Not content with changing the face of energy storage, the inventor of lithium-ion batteries is now heading up a team of scientists who claim to have found a new cathode material.
John Goodenough led an international team of scientists at the University of Texas, US who identified the mineral eldfellite ( NaFe(SO4)2) as a potential cathode for a sodium-ion battery.
The breakthrough was published in a online paper called Eldfellite, NaFe(SO4)2: an intercalation cathode host for low-cost Na-ion batteries
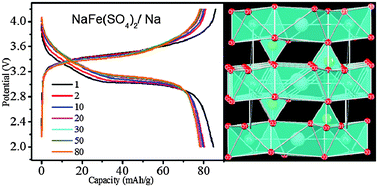
Eldfellite (NaFe(SO4)2) is comprised of Na+ and Fe3+ layers separated at a fixed distance by SO42- polyhedra.
These fixed spaces allow the diffusion of sodium ions, meaning the material is capable of realising 80% of its theoretical maximum specific capacity.
‘The problem is the size of the sodium ion,’ Preetam Singh, one of the scientists behind the research, told Chemistry World.
‘You can’t simply duplicate lithium-ion technology by directly substituting lithium for sodium in the chemical structures of various cathode and electrolyte materials, because the sodium–sodium interaction is much stronger and so you cannot get good rate performance.”
Lithium-ion batteries have transformed the world, making mobile phones a must have gadget and electric vehicles a popular accessory for eco-minded individuals.
However, the technology is hampered by a finite supply of lithium making production costs high, which in turn means large scale applications and even EVs are limited in their mass-market appeal.
Researchers have been attempting to tackle this problem by replacing lithium with sodium, with companies such as Aquion Energy and Faradion focusing on sodium-ion batteries.
Sodium-ion can also be 100% discharged and is safe to transport, unlike its lithium counterpart.