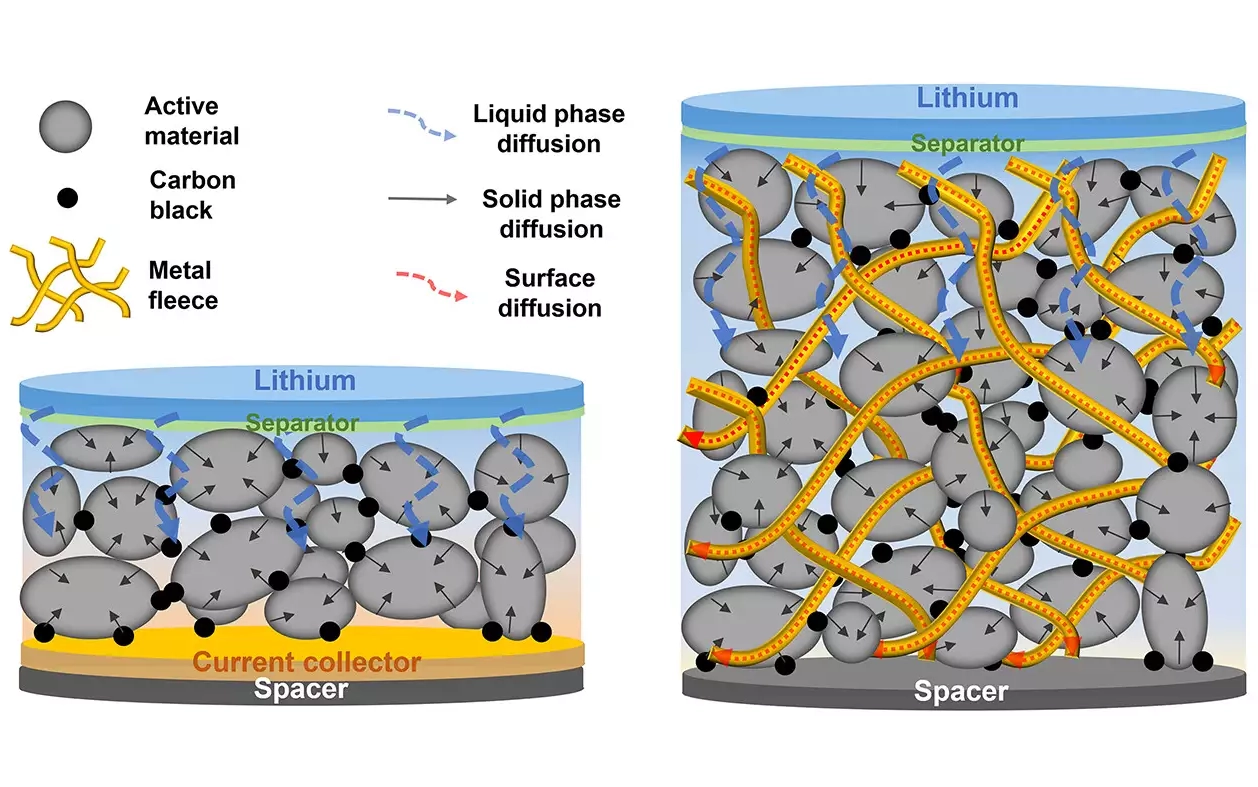
A discovery by researchers at the Max Planck Institute for Medical Research in Heidelberg could give lithium batteries a significant energy boost. The team, led by Max Planck Director Joachim Spatz, has discovered that metal fleeces used in place of foils for battery electrodes significantly accelerate the charge transport of metal ions.
Existing lithium-ion batteries consist of layers where the active material is applied to a thin aluminium or copper foil ~0.01mm thick. The foil only serves to transport the current to and from the electrode. The use of foils in existing batteries means the active material layer must be thin, for fast charge/discharge, or thicker for capacity. The lithium ions have to travel through the liquid electrolyte into the porous active material.
Using a fleece makes it possible to build significantly thicker electrodes than is standard today. Roughly half of the contact metal and other materials that do not contribute to energy storage can be saved. This made it possible for researchers to significantly increase the energy density in batteries.
“Supplying a material with charge via two-dimensional layers is in no way efficient,” says Joachim Spatz, pointing to the example of nature: It supplies organisms via a three-dimensional network of vessels. “That is the goal of our technology: A 3D supply network for charge carriers that can be used to charge and discharge batteries efficiently.”
The fleece is produced from metal threads that are only a few hundredths of a millimetre thick. Rather than the complex process of applying thin layers of active material to the contact foils, often with the use of toxic solvents, the fleece can be dry filled with the active material in powder form. “With dry filling, we can probably save 30–40% of production costs, and the production facilities need a third less space,” says Joachim Spatz. The result is fleece electrodes that are significantly easier and cheaper to manufacture.
In a study published in the journal ACS Nano, the Heidelberg team has shown how electrodes can be produced at least ten times thicker than is usual today and still be charged and discharged quickly. “The basis for this is a previously unknown mechanism that we discovered in ion transport in electrodes,” says Joachim Spatz.
The researchers demonstrated that lithium ions strip off their molecular shell on a copper surface, deposit themselves there and form an electrical double layer with electrons that accumulate under the metal surface, known as the Helmholtz layer. “Using a specially developed measurement setup and theoretical calculations, we have shown that the lithium ions move through the Helmholtz layer around 56 times faster than through the electrolyte,” says Joachim Spatz. “Metal surfaces are therefore a kind of motorway for the metal ions.”
By inserting the active material into the fleece, the contact area is greater, and the amount of copper required is half that of a foil. The bottom line is energy density that is up to 85% higher.
Image: Today’s lithium-ion battery cells are very complex in their structure and manufacturing process (left). Metal fleeces (batenefleeceTM) as electrical contacts simplify the design and manufacture of a battery and reduce the share of passive materials. This makes batteries cheaper and more powerful (right).