A collaboration between US and German scientists has developed a dual-ion battery they claim is safer, cheaper and more sustainable than established energy storage systems.
Researchers have evaluated in laboratory prototype cells a chemistry combining graphite/zinc metal with an aqueous electrolyte that functions as an active material.
The teams are from the US Pacific Northwest National Laboratory (PNNL) and the Battery Research Center of Germany’s Münster Electrochemical Energy Toechnology (MEET).
The detailed results have been published in the journal Advanced Energy Material.
The dual-ion battery can operate at ≈2.3–2.5V, achieving >80% capacity retention after 200 cycles and deliver ≈110mAh/g at a charge/discharge current of 200mA/g.
A capacity of nearly 60 mAh/g is achieved at a charge/discharge current of 5000mA/g.
Further R&D of electrolyte modification and interphase stabilisation is now being conducted to improve cycle life.
A spokesperson from MEET told BEST it was hard to predict costs at this stage because there were many possibilities for material (anode/cathode) and component (electrolyte solvents/salts) variations, which needed to be specifically evaluated in terms of performance and cost advantages.
The spokesperson said: “Some points to consider for cost calculations when comparing dual-ion batteries to state-of-the-art lithium-ion batteries (LIBs) are:
Layered oxide cathode (LIBs) can be replaced by low-cost graphite materials (natural or artificial) as cathode material, which can also be processed by an aqueous route (without toxic organic solvents).
“Zinc metal can be applied as low-cost anode material (compared to Li metal). The electrolyte composition (salt/solvent) still needs to be optimised and will significantly impact the cost, in particular electrolyte salts such as LiTFSI/LiFSI have a negative impact on cost.”
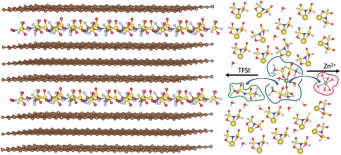
Image: Simulated anion intercalation into graphite and cation solvation structure in “water‐in‐bisalt” electrolyte