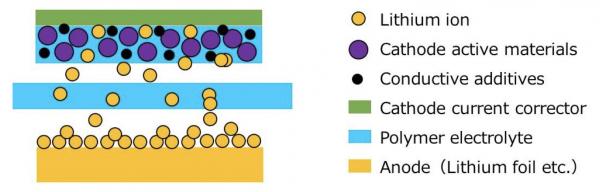
Nippon Shokubai, based in Osaka, Japan, has succeeded in improving the electrolyte performance for solid polymer batteries.
Solid-state batteries using polymer electrolyte have features such as long life and high safety, but the polymer electrolyte has poor lithium ion conductivity so it is necessary to heat the battery to more than 50°C.
The newly developed polymer electrolyte shows high lithium conductivity performance at near to room temperature, paving the way for new solid-polymer battery applications.
The firm initially developed a type of solid electrolyte consisting of polyethylene oxide for a lithium polymer battery and started commercial production around 2013. Ionic conductivity of polyethylene oxide polymer electrolyte is generally lower than that of non-aqueous electrolyte for lithium-ion batteries, with a lithium ionic transport number of 0.1 to 0.2.
Several studies to improve the lithium ionic transport number of polymer electrolytes have been reported, but most of them are not able to improve the performance because of the low ionic conductivity.
The company’s newly developed electrolyte film has ionic conductivity almost the same as, and a lithium ionic transport number more than five times higher than, conventional polyethylene oxide electrolyte film.
The electrolyte film has oxidation-reduction stability for lithium metal and 4V-class cathode active materials.
The laminated cell battery fabricated using this technology exhibited far higher discharge characteristics than polyethylene oxide-based polymer.
The dramatic improvement in battery performance leads to many potential benefits, including shorter charging time, higher energy density, and reduction of battery-heating devices.