A Chinese research team, led by Yingchun Niu and Senwei Zeng, has developed a nitrogen-boron doped electrode for the iron-chromium redox flow battery (ICRFB).
ICRFBs use low-cost, non-toxic iron and chromium redox pairs as catholyte and anolyte in hydrochloric acid solution. The carbon cloth (CC) electrode in ICRFBs is the main site where the electrochemical reaction occurs.
Although CC has the merit of low-cost, corrosion resistance and wide operating potential range it has the drawbacks poor electrochemical reactivity, which has hindered the commercial development and application of ICRFBs.
The team from State Key Laboratory of Heavy Oil Processing, China University of Petroleum Beijing published its research in Green Energy and Intelligent Transportation.
The paper explains that better physicochemical properties of the carbon-based electrodes are obtained through various modification methods including ontology modification, loaded nanomaterials, loaded metals and heteroatom doping.
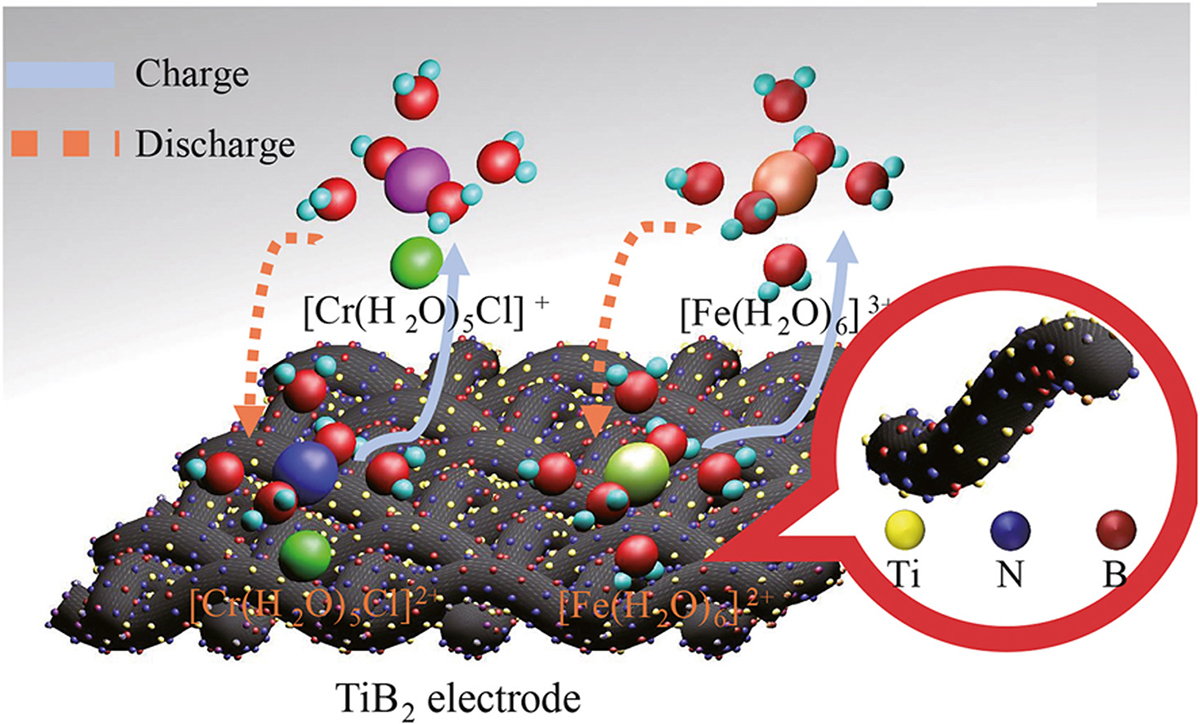
The team proposed the boron-nitrogen doped coupled TiB2 catalyst carbon felt electrode and successfully applied it to ICRFB. Boron and nitrogen were doped into carbon fibre through high-temperature calcination.
The N-B co-doped coupled TiB2 composite electrodes were prepared by deposition of TiB2 catalysts, which introduced abundant boron and nitrogen co-functionalised defects on the carbon nanofibers surface and sped up the redox reaction of ICRFB.
The newly fabricated N-B co-doped co-regulation Ti composite electrode nearly doubles the specific surface area compared with the original electrode (1.21m2/g vs 0.73m2/g).
The energy efficiency of the T-B-CC equipped battery at 140mA/cm2 was substantially increased to 82.7% in contrast with the cell based on thermo-treated carbon felt (76.17%).
Furthermore, the prepared electrode also demonstrated a higher discharge capacity (1,990.3mAh vs 1,155.8mAh) within 50 charge/discharge cycles.
The team concludes: the composite electrode has marvellous catalytic activity and excellent conductivity. It provides plentiful reactive sites and accelerates the reactants’ diffusion rate, in addition to facilitating electron transfer and strengthening the physicochemical properties of the electrode. The N-B co-doped coupled TiB2 composite electrode, with excellent rate and cycling performance, is expected to be widely used in ICRFB.