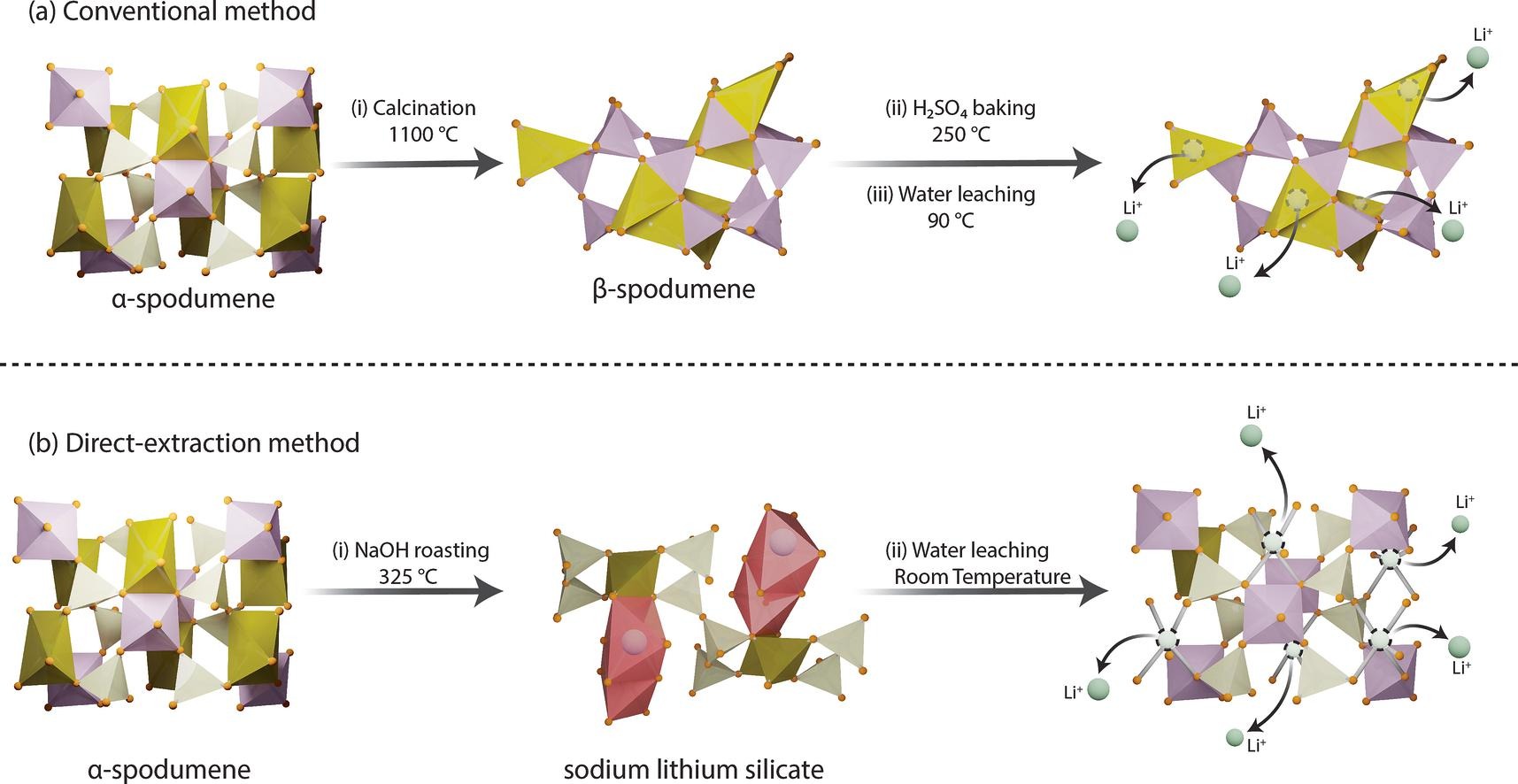
Researchers at Penn State University claim to have developed a high-efficiency method for extracting lithium metal from the mineral α-spodumene, which contains lithium aluminium silicate.
This patent-pending method is said to use less water and reduce energy consumption.
The researchers, led by Mohammad Rezaee, converted the pyroxine mineral α-spodumene to water-soluble phases using a relatively low temperature roasting using sodium hydroxide.
This NaOH roasting process was in two-stages at 325°C and water leaching was done at room temperature, which the researchers claim extracted over 99% of the lithium.
The water leaching was an exothermic reaction and the researchers said that it showed fast kinetics, with the most lithium recovery within one minute.
The authors also explored microwave (MW) heating to take advantage of the bipolar characteristics of NaOH and enhance energy efficiency during the roasting process.
This method was compared to a sequential water and acid leaching of NaOH roasted α-spodumene, which used the conventional heating method. It recovered 71% and 17% of Li respectively with a total recovery of 88%.
The researchers believe the conventional method to be complex and energy intensive, especially with the high-temperature calcination and sulphuric acid baking process. It also releases a lot of greenhouse gas emissions.
Mohammad Rezaee, lead researcher, told Tech Xplore: “Lithium powers the technologies that define our modern lives … but its extraction must also be financially responsible. Our research shows that we can extract lithium, and other critical minerals, more efficiently while drastically reducing energy use, greenhouse gas emissions and waste that’s difficult to manage or dispose of.”
Image: A graphical abstract comparing the two lithium extraction methods. Credit: Penn State University.