Researchers at Sandia National Laboratories have designed a new class of molten sodium batteries, operating at much cooler temperatures, for grid-scale energy storage.
The new battery design was shared in a paper published in the scientific journal Cell Reports Physical Science.
While traditional molten sodium batteries operate at around 300°C, Sandia’s new molten sodium-iodide battery operates at a much cooler 110°C.
Leo Small, the lead researcher on the project, said: “We’ve been working to bring the operating temperature of molten sodium batteries down as low as physically possible.
“There’s a whole cascading cost savings that comes along with lowering the battery temperature.
“You can use less expensive materials, the batteries need less insulation and the wiring that connects all the batteries can be a lot thinner.”
The battery consisted of a Sn-saturated Na anode, a Sn-coated (170 nm thickness) NaSICON separator, NaI-GaCl3catholyte, and thermally activated carbon felt cathode current collector.
The latest development, using a revolutionary high-voltage NaI-GaCl3 (sodium iodide and gallium chloride) molten salt catholyte, enables stable electrochemical cycling in a molten Na-NaI battery.
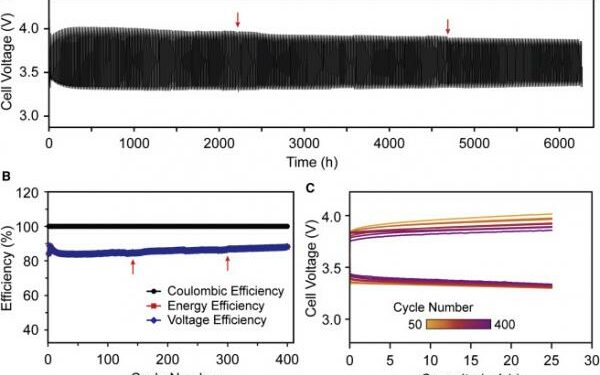
The test battery has operated in the laboratory at the dramatically reduced temperature of 110°C for more than eight months and was cycled more than 400 times.
Throughout cycling, the battery cycling program was periodically stopped for a few hours to accommodate other experiments in the shared oven space.
This periodic stoppage appears to have had limited effect on the battery performance, as seen by a <0.5% average drop in energy efficiency in the cycle following the stoppage, which would be recovered within a few cycles.
The batteries were twice cooled to room temperature, first for one month and second for one week.
The ability to freeze the charged battery and discharge it at a later time is especially advantageous for long-duration grid-scale energy storage, in which a battery may be charged during one month, and then discharged several months later.
Erik Spoerke, a materials scientist who has been working on molten sodium batteries for more than a decade, said: “This is the first demonstration of long-term, stable cycling of a low-temperature molten-sodium battery.
“The magic of what we’ve put together is that we’ve identified salt chemistry and electrochemistry that allow us to operate effectively at 110°C.”
Commercial molten sodium batteries have lifetimes of 10-15 years, significantly longer than standard lead-acid batteries or lithium-ion batteries.