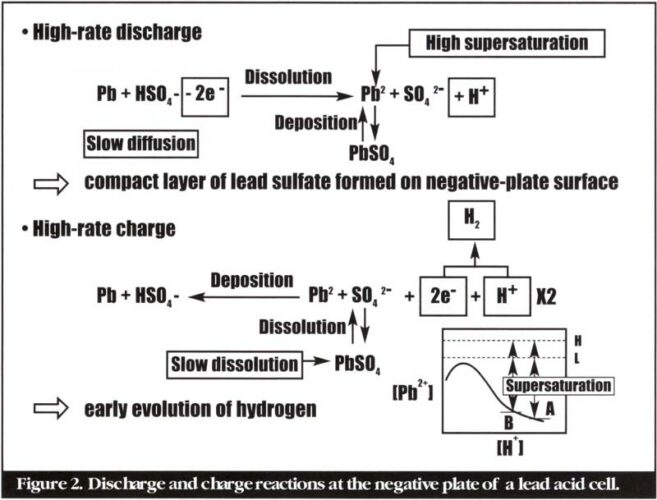
Lan Lam, Pat Moseley and David Rand de-mystify negative-plate sulphation – the phenomenon that must be addressed in order for valve-regulated lead-acid batteries to enter hybrid electric vehicle markets.
Future automobile electrical systems will require batteries to function in a regime that is beyond the capabilities of standard automotive units. The move towards such systems has already begun, with the arrival on the world's roads of around 200,000 hybrid electric vehicles (HEVs).
To date, all commercial HEVs have been equipped with nickel–metal-hydride batteries that operate in long strings of . . .
to continue reading this article...
Sign up to any Premium subscription to continue reading
To read this article, and get access to all the Premium content on bestmag.co.uk, sign up for a Premium subscription.
view subscription optionsAlready Subscribed? Log In