Frank Lev reports on arguments that electric vehicles need more energy-dense batteries.
Lotus recently announced it could delay the launch of its Type 135 electric sports car until smaller, lighter batteries become available. The company reckons the current lithium-ion batteries do not allow their EVs to compete successfully with internal combustion engine (ICE) sports cars like Elise. Lotus head of design Ben Payne thinks that more energy-dense solid-state batteries could enable them to build compact, light electric sports cars.
Payne’s thoughts are shared by Volkswagen Group (VW), whose battery company, PowerCo (PCo), has partnered with one of the leading solid-state battery technology developers, QuantumScape (QS), to expedite the commercialisation of QS’s solid-state battery technology.

Partially owned by VW, QS is one of the significant developers of a solid-state battery with demonstrated fast-charging capability and high energy density. Tested by PCo, the QS 24-layer A0 battery prototypes (Fig 1) endured 1,000 total cycles, retaining up to 95% state of charge. At discharge rate C/3 to C/2, cathode loading 3.1mAh/cm2, temperature of 25°C, and stack pressure of 3.4 bar, the prototypes’ energy density was more than 1,000Wh/L.
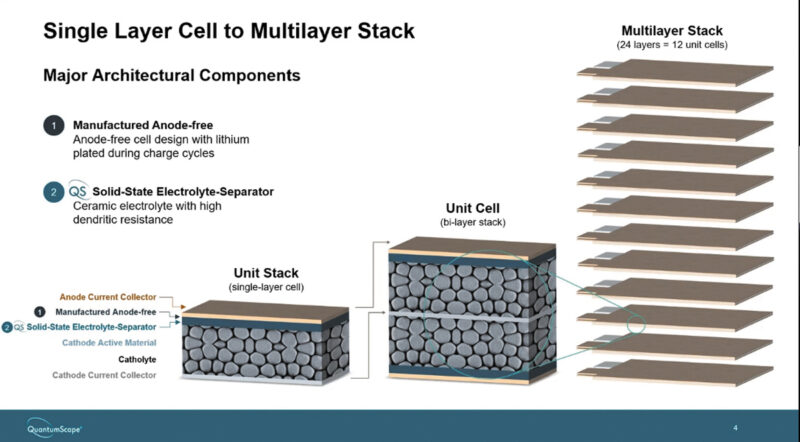
The technology uses a ceramic electrolyte and lithium-metal (Li-metal) anode formed in situ at charging. The company plans to upgrade single-layer cells to multi-layer cells and start low-volume B-sample prototype production in 2024. Table 1 shows the QS’s production roadmap. The company said it has has been producing multilayer cells for many years.

Thomas Schmall, VW board member for technology, said: “EVs are the future of mobility, and this agreement with QS will ensure VW’s global fleet has access to this groundbreaking battery technology for years to come. We are committed to driving the industry forward to ensure our EVs set the benchmark for excellence and sustainability.” The agreement replaces the previous joint venture between the two companies to manufacture solid-state batteries. It combines QS’s technology with PCo’s production and financial prowess.
Improving EV range
This article aims to highlight the essentials of QS technology to reflect the principles of the solid-state battery philosophy and design for a better understanding of its advantages and challenges compared with incumbent lithium-ion batteries. The widely used lithium-ion batteries typically consist of NMC or LFP cathodes, graphite anodes (with or without silicon) and liquid electrolytes.
They work on the lithium intercalation principle, which has not changed since their introduction to the market in 1991. Although outstanding in their significance for electronic, automotive and BESS industries, these batteries are at the end of their theoretical capabilities regarding energy density, fast charging and safety.
One of the limiting factors in these batteries is the graphite anode material. A typical lithium-ion battery with a graphite anode has an energy density in the ballpark of 650Wh/L. For instance, the Tesla Model Y circa 2020 with Panasonic 2170 batteries has 680Wh/L. A lithium-ion battery with a silicon anode may have up to 800Wh/L. However, to compete and eventually replace ICE vehicles, EVs need at least 1,000Wh/L batteries with improved life, safety and recharge time.
Li-metal a game changer
Li-metal has always been considered the ‘Holy Grail’ anode material due to its high volumetric (2,046mAh/cm3) and gravimetric (3,862mAh/g) capacities and a low reduction potential (-3.04V vs. standard H2 electrode). In a striking difference to lithium-ion batteries with graphite and silicon anodes that intercalate lithium, a Li-metal anode is unincumbered by any host materials, thus reducing the weight and volume of the anode.
“This is why we started this solid-state journey in the first place – so we could use Li-metal,” explained Prof Helena Braga, a well-known researcher who worked with Nobel Prize winner John Goodenough on solid-state batteries a decade ago.
However, the caveat to applying these anodes is the high reactivity of Li-metal with nearly all aprotic organic solvents of liquid electrolytes promoting dendrite growth and an unstable solid electrolyte interphase (SEI) formation.
Solid electrolytes matter
The dendrites caused by such a reaction are conducive to volatile fires due to the short-circuiting of battery electrodes. In contrast, solid electrolytes are not flammable, do not react with Li-metal and are impervious to dendrite penetration, providing a viable solution for the safe use of Li-metal anodes.
Solid-state batteries (except semi-solid) have no liquid or gel electrolytes because they use the ionically conductive solid electrolyte, which also works as a separator to keep the electrodes from shorting. Not only do solid-state electrolytes resist fire, but they also do not leak, making solid-state batteries much safer than those with liquid electrolytes.
QS solid-state technology is only partially solid
In contrast to a pure solid-state technology, which, by definition, must not have liquid components, the QS battery belongs to the semi-solid battery technology in which the cathode uses a liquid electrolyte, whereas the anode uses a solid one.
The indisputable advantage of such an approach is its simplicity, low impedance and reliability of the cathode-electrolyte interface. In the solid-state design, such an interface notoriously suffers from high impedance, requiring high stack compression and other remedies to reduce it. Liquid electrolytes do not have such complexities. In QS’s words, “a liquid electrolyte (catholyte) is better suited for the voltage and transport requirements of the cathode.”
However, the flip side of such a design is the flammability of the liquid electrolytes. To some extent, QS improved safety by using a gel electrolyte comprising a solvent, lithium salt and a PVDF-HFP polymer to make it semi-solid instead of liquid. Gel electrolyte, known as lithium-polymer, is considered semi-solid because it has both solid and liquid properties. It has been successfully used in commercial lithium-polymer batteries like Blue Solutions. Although less conductive than liquid electrolytes, the gel is less violent because the polymer immobilises its explosive solvent vapour.
The enabler
The quintessential feature of QS’s technology is its solid-state ceramic electrolyte, which also serves as the separator and enables the use of a Li-metal anode because it prevents dendrite penetration.
The QS solid-state separator is a solid electrolyte material capable of high conductivity, Li-metal stability, dendrite resistance and low interfacial impedance. Such design enables the battery to attain high energy density, fast charge and a long life. Unlike some commercial lithium-ion battery separators, the QS ceramic separator is non-combustible. It is made from abundant materials using a cost-effective continuous manufacturing process at commercial volumes.
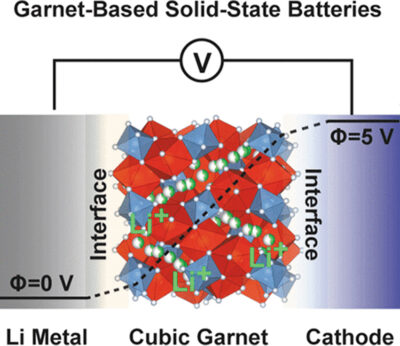
QS researchers identified lithium-stuffed garnet electrolytes (Fig 2) as a crucial component for their solid-state battery design due to their high ion conductivity, electric insulating properties, and chemical compatibility with Li-metal anodes.
Moreover, solid-state lithium-stuffed garnet electrolytes can work as battery separators, making them thinner and lighter than conventional ones. Formulated by QS garnet-type (LLZO), solid-state electrolytes have exceptionally high ionic conductivity (10–3 to 10–4 S/cm) and good chemical stability against Li-metal, offering an excellent opportunity for solid-state Li-metal batteries. QS patent literature characterises its garnet electrolyte/separator by the following chemical formula: Lix1La3Zr2O12 y(Al2O3), wherein 3≤x1≤8 and 0≤y≤1.
Electrolyte separator
The calcined lithium-stuffed garnet powder is a primary precursor for the QS electrolyte separator. Calcining is typically a chemical reaction between solid components used for ceramic and sintering processes. Sintering is a high-temperature process used for material solidification and mechanical stability.
To prepare the calcined lithium-stuffed garnet powder of Li7La3Zr2O120.5Al2O3 molar ratio, QS mixes lithium hydroxide (LiOH), aluminium nitrate [Al(NO3)39H2O], zirconia (ZrO2) and lanthanum oxide (La2O3) in specific proportions. The mixture with added ZrO2 milling media and a dispersant is further milled to a d50 100nm to 5μm particle size.
Next, the powder is separated from the milling media and calcined at about 900°C for approximately six hours in a controlled oxidising atmosphere. The calcination process incinerates the residual solvents, dispersants, binders, and surfactants to form the calcined lithium-stuffed garnet.
The next step is mixing the powder with a plasticiser, a polyvinyl butyrate binder, and a butanol solvent to produce a slurry containing about 80% of the solids. The slurry is then cast to make 10–200μm thin films of calcined but unsintered lithium-stuffed garnet. These dry calcined films are called green films, not because their colour is green, but because they are unsintered.
The next step is sintering, conducted at 1200°C in an Argon/H2O atmosphere for six hours to achieve the desired density of about 4.7g/cm3. QS has developed a process of adding metal powder to the green film, which turns into a metal current collector during sintering. The precursor powder from which QS makes the green films contains a certain percentage of ceramic particles mixed with a binder.
The binder assists in sintering the ceramic particles to achieve a consistent film of the required thickness and is removed from the film by heating during the sintering process. The final step is annealing the film at 350°C to eliminate contamination and improve its ionic conductivity. After sintering and annealing, the green film can be used as a solid electrolyte separator.
Numerous advantages
One of the striking features of QS technology is that the battery is assembled without an anode per se, which contrasts sharply with traditional batteries where the anode is one of the principal battery components. Remarkably, the Li-metal anode forms in situ on the first charge in the QS battery. As the battery is charged, the lithium ions travel from the cathode through the separator towards the negative current collector, forming a thin layer of Li-metal that acts as an anode.
Nearly all lithium that had formed the anode returns to the cathode during the discharge cycle, eliminating the need for any host material (like graphite) and reducing the weight and volume of the anode. The QS anode-free battery is also called a zero-excess Li-metal battery, with all active lithium-ions initially stored in the cathode material.
According to the company, the anode-free design reduces manufacturing costs as well.
High charge capacity
QS has also introduced a new cathode with a high packing density of the active material. The material had undergone field testing in QS’s two-layer cells and demonstrated a charge capacity of 5mAh/cm2, much better than the previous capacity of 3.1mAh/cm2. The new cathodes have higher energy density than the most advanced 2170 battery cathodes with 4.3mAh/cm2.
QS explained: “The higher energy density is primarily due to higher-loading cathodes (i.e. packed with more active material) and more efficient packaging that optimises the materials and space within the cell.” Also, the improvements include “tighter internal margins, thinner current collectors, and a slimmer design.”
In its Letter to Investors, QS wrote, “We are happy to report that we have shipped high cathode-loading unit cells to multiple automotive partners, which aligns with our development roadmap. This is an important milestone because this level of cathode loading is close to our commercial-intent cathode design for energy-dense cells and represents a significant step toward delivering a commercial product. In our view, when combined with the 24-layer capability we have already shown in our A0 prototype cells and other planned improvements, these shipments validate our ability to achieve industry-leading energy and power performance for our first commercial product.”
Advanced production equipment
The forthcoming QSE-5 cells will consist of 24 layers (Fig 3) and use solid electrolyte separators produced on the equipment it calls ‘Raptor’ to handle the most challenging and expensive manufacturing process – the separator heat treatment. The ‘Raptor’ can run the heat process fast and efficiently, tripling the production capacity of the B0 version of the QSE-5 cells.
QS has not elaborated the name for its highly sophisticated equipment, but a good guess is that it represents the first deployment of a disruptively faster separator heat treatment process. The next generation of the ‘Raptor’ is aimed at mass-producing a solid-state battery at gigawatt scale.
Five principles

Jagdeep Singh, founder and former CEO of QS, claims that QS batteries will effectively deliver on five fundamental consumer needs that have thus far prevented electric vehicles from surpassing the 2% of new US auto sales: lower costs, greater range, shorter charging times, longer total lifetime on the road and improved safety. “Any battery that can meet these requirements can really open up the 98% of the market in a way you cannot do today,” said Singh.
Faster charging
The QS batteries can be recharged from the 10% to 80% state of charge in less than 15 minutes – a significant improvement compared to the 26 minutes required to recharge the latest VW ID.3 GTX electric car. Frank Blome, CEO of VW Group’s PowerCo battery cells subsidiary and a board member at QS, said: “Solid-state batteries will be able to charge to the 80% capacity in as little as 12 minutes.” He added the solid-state battery will deliver about 30% more range than a liquid-type battery of the same size and weight.
This means that the existing VW ID.3 GTX, specified to cover 605km on a single charge, will be capable of 780km.

According to QS, the first commercial product will be a battery cell with the designation QSE-5 (Fig 4) and a charge capacity of around 5Ah with the following characteristics:
- higher-loading cathodes of up to 5.6mAh/cm2
- anode-free Li-metal design
- 5Ah FlexFrame format.
QSE-5 will have an energy density of over 800Wh/L. The specific energy is still the company’s secret.
QS projects the cost of its solid-state batteries to be in the same ballpark as the incumbent EV batteries owing to the low price of self-forming anodes, materials used in the ceramic electrolyte separator and the efficient manufacturing.
Exceeded expectations
The extended QS battery tests in PCo’s battery laboratories in Salzgitter produced results that exceeded expectations. The endurance tests demonstrated over 1,000 charging cycles, which equates to approximately 500,000km of driving – 20% more than QS’s previous tests. PCo CEO Frank Blome said: “These are very encouraging results that impressively underpin the potential of the solid-state cell. The final result of this development could be a battery cell that enables long ranges, can be charged super-quickly and practically does not age. We are convinced of the solid-state cell and continue working at full speed with our partner QuantumScape towards series production.”
Blome continued: “In the standardised test procedures for newly developed battery cells, robustness is considered the most important criterion. The industry-standard targets for this development phase are 700 charging cycles and a maximum capacity loss of 20%. QS’s solid-state cell significantly exceeded these specifications in the latest test. The cell also met the requirements for other test criteria such as fast-charging capability, safety and self-discharge.”
Commenting on the test results, Jagdeep Singh said: “These results from the Volkswagen Group’s PowerCo testing make clear that QuantumScape’s anodeless solid-state lithium-metal cells are capable of exceptional performance. While we have more work to bring this technology to market, we are unaware of any other automotive-format lithium-metal battery that has shown such high discharge energy retention over a comparable cycle count under similar conditions. We are excited to work closely with the Volkswagen Group and PowerCo to industrialise this technology and bring it to market as quickly as possible.”
In the summer of 2023, QS hired Dr. Siva Sivaram, a specialist in semiconductor manufacturing. QS expects to benefit from Dr. Sivaram’s experience, as solid-state battery production and semiconductor manufacturing have much in common. Thus far, QS is on schedule with the production of the new solid-state battery cells, which is supposed to start in 2024.
The PCo labs in Salzgitter, Germany might become the place for six vertically integrated battery manufacturing centres. PCo plans envisage that “each factory will operate 100% on electricity from regenerative sources and will be designed for future closed-loop recycling. The Salzgitter production centre will be followed by another in Valencia and three others at undisclosed European locations.
The Salzgitter factory alone will build enough batteries for 500,000 EVs per year. There are also plans for building gigafactories in North America. QS projects revenues of $14 million in 2024 and $39 million in 2025. It aims to ramp up to $275 million in 2026 and $3.21 billion in 2027. Shares of the company rose more than 40% after VW confirmed that QS’s batteries successfully passed the endurance test, thus acknowledging that the long journey to commercialisation has begun.

Rival solid-state technologies
Several other developers of solid-state batteries reported using anode-free cell technologies. The ION Storage Systems (ION) announced that its cells achieved 800 cycles. Similar to QS, these batteries use, according to Dr. Nicholas Hudak, Director of R&D at ION “the combination of the next-generation ceramic electrolyte and a unique anode-free architecture.”
In contrast with QS, the ION batteries do not require stack compression. Typically, Li-metal solid-state batteries must use stack compression to increase cycling stability, reduce dendrite formation and increase coulombic efficiency. However, such compression requires clamping devices impacting gravimetric and volumetric energy densities. Understandably, the free-of-compression ION technology is viewed as very promising.
American start-up, Our Next Energy (ONE) has developed in collaboration with BMW anode-free solid-state batteries with an energy density of 1,007Wh/L. The ONE’s energy density appears on a par with QS’s.
Both companies acknowledged that ‘anode-free’ is a somewhat ambiguous description of their batteries, which still have cathodes and anodes. However, similarly to QS, they use Li-metal anodes with no graphite.
The concluding statement
As a mature developer of solid-state battery technology, QS exemplifies the synergy of solid-state ceramic electrolytes and a Li-metal anode-free design to achieve a truly remarkable performance. While other developers use similar designs, showing that QS is not the only fish in the pond, QS is confidently moving forward to the production stage.
Despite QS’s outstanding achievements, purists may criticise the QS technology, pointing out that it is only partially solid-state as it has a liquid electrolyte. Who cares? I do not, as long as they meet the objective of reliably using the Li-metal anode and achieving the targeted performance goals of 1,000Wh/L, 1,000 cycles and 12 minutes’ recharging time. That matters the most to the automotive OEMs, and if everything goes according to QS and PCo plans, their Li-metal batteries will be under the hood of VW vehicles soon enough.