An accidental discovery by chemical engineers at Drexel University has revealed a method for introducing sulfur into lithium-ion batteries that delivers more than 4,000 recharges.
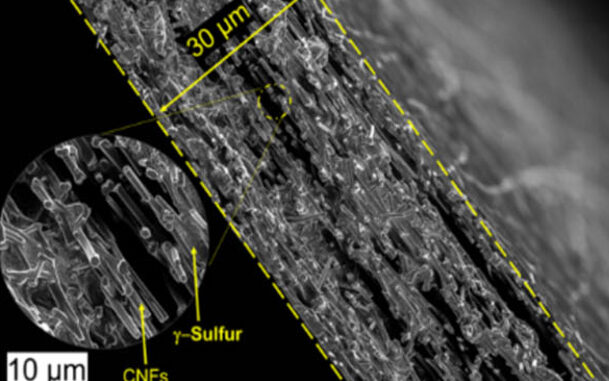
Researchers in Drexel’s College of Engineering in the US confined sulfur in a carbon nanofiber cathode substrate using a vapor deposition technique to prevent polysulfide formation.
While the process failed in embedding the sulfur within the nanofiber mesh, subsequent tests revealed the sulfur cathode performed well, and continued to do so without causing shuttling.
The team found that during the process of depositing sulfur on the carbon nanofiber surface— changing it from a gas to a solid — it crystallised to form a slight variation of the element monoclinic gamma-phase sulfur.
This chemical phase of sulfur, which is not reactive with the carbonate electrolyte, had previously only been created at high temperatures in laboratories and has only been observed in nature in the extreme environment of oil wells.
Rahul Pai, a doctoral student in the Department of Chemical and Biological Engineering and co-author of the research, said: “At first, it was hard to believe that this is what we were detecting, because in all previous research monoclinic sulfur has been unstable under 95 degrees Celsius.
“In the last century there have only been a handful of studies that produced monoclinic gamma sulfur and it has only been stable for 20-30 minutes at most.
“But we had created it in a cathode that was undergoing thousands of charge-discharge cycles without diminished performance— and a year later, our examination of it shows that the chemical phase has remained the same.”
Drexel’s Vibha Kalra, George B. Francis Chair professor in the College’s Department of Chemical and Biological Engineering, led the research.
The research was published in the journal Communications Chemistry. Read the full paper here:
Lithium-sulfur battery breakthrough
Developing a method that produces and stabilises a rare form of sulfur that functions in carbonate electrolyte not only makes sulfur batteries commercially viable, but they would have three times the capacity of Li-ion batteries and last 4,000 cycles.
The method could deliver a way to sidestep the obstacles that have subdued lithium-sulfur batteries in the past, finally pulling the sought-after technology within commercial reach.
Kalra said: “Sulfur has been highly desirable for use in batteries for a number of years because it is earth-abundant and can be collected in a way that is safe and environmentally friendly and, as we have now demonstrated, it also has the potential to improve the performance of batteries in electric vehicles and mobile devices in a commercially viable way.”
The challenge of introducing sulfur into a lithium battery with commercially friendly carbonate electrolyte has been an irreversible chemical reaction between intermediate sulfur products, called polysulfides and the carbonate electrolyte.
Because of this adverse reaction, previous attempts to use a sulfur cathode in a battery with a carbonate electrolyte solution resulted in complete failure of the battery after one cycle.
Lithium-sulfur batteries have already demonstrated exceptional performance in experimental settings using an ether electrolyte — rather than carbonate — because ether does not react with polysulfides.
However, these batteries are not commercially viable because the ether electrolyte is highly volatile and has components with a boiling point as low as 42oC.
Kalra said: “In the past decade, the majority of Li-S field adopted ether electrolytes to avoid the adverse reactions with carbonate.
“Then over the years, the researchers deep-dived into enhancing performances in ether-based sulfur batteries by mitigating what is known as polysulfide shuttle/diffusion — but the field completely overlooked the fact that the ether electrolyte itself is a problem.
“In our work, the primary objective was to replace ether with carbonate, but in doing so we also eliminated polysulfides, which also meant no shuttling, so the battery could perform exceptionally well through thousands of cycles.”
Kalra’s team had previously produced a carbon nanofiber cathode that slowed the shuttle effect in ether-based lithium-sulfur batteries by curtailing the movement of intermediate polysulfides.
The discovery came when the team tried to eliminate this polysulfide formation.
Kalra said: “While we are still working to understand the exact mechanism behind the creation of this stable monoclinic sulfur at room temperature, this remains an exciting discovery and one that could open a number of doors for developing more sustainable and affordable battery technology,”
Curtailing raw material supply concerns
Using sulfur as a lithium-ion cathode alleviates the need for cobalt, nickel and manganese— materials that are facing supply concerns as well as humanitarian issues in the case of cobalt.
Sulfur, however, is abundant and exists in vast quantities— a key bonus for the US’ battery industry.
The Drexel team hopes the breakthrough will allow researchers to move forward in examining replacements for the lithium anode, which could include more earth-abundant options such as sodium.
Kalra said: “Getting away from a dependence on lithium and other materials that are expensive and difficult to extract from the earth is a vital step for the development of batteries and expanding our ability to use renewable energy sources.”
In addition to Kalra and Pai, Maureen Tang, PhD, an associate professor; and Arvinder Singh, PhD, who was a postdoctoral researcher; all in Drexel College of Engineering’s Department of Chemical and Biological Engineering, contributed to this research.