Scientists at Brookhaven National Laboratory have identified the primary cause of failure in a state-of-the-art lithium-metal battery.
Using high-energy x-rays, scientists followed the cycling-induced changes at thousands of different points across the battery to identify depletion of the liquid electrolyte as the primary cause of failure.
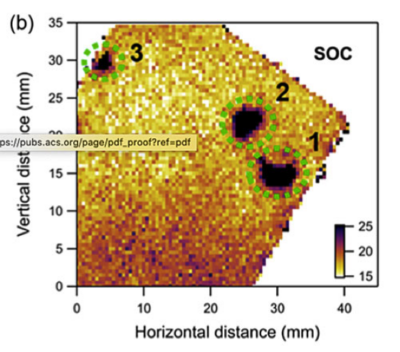
The team mapped the variations in performance, and at each point used the x-ray data to calculate the amount of cathode material and its local state of charge.
These findings, combined with complementary electrochemical measurements, enabled them to determine the dominant mechanism driving the loss of battery capacity after many charge-discharge cycles.
The findings were reported in the journal Chemistry of Materials.
The electrolyte transports lithium ions between the rechargeable battery’s two electrodes during each charge and discharge cycle.
Since 2017, the Battery 500 Consortium— a PNNL-led and DOE-sponsored group of national laboratories and universities— has been working to develop lithium-metal anodes with an energy density three times higher than current automotive batteries
Lithium metal is very reactive and degrades as the battery cycles. The degradation reactions consume the electrolyte over time.
Early on in their development, high-energy-density lithium-metal anodes had a very short lifetime, typically 10 cycles or less. Battery 500 Consortium researchers improved this lifetime to 400 cycles in 2020, and are aiming for lifetimes of 1,000 cycles or more to meet electric vehicle needs.
State-of-charge mapping showed ‘hotspots’ where the test cell performance was reduced.
The finding suggested the capacity loss was due to partial destruction of the liquid electrolyte and this was confirmed in follow-up experiments on coin cells that were designed to intentionally fail through electrolyte depletion.
Brookhaven chemist professor Peter Khalifah said: “Electrolyte depletion was the failure mechanism most consistent with the synchrotron x-ray and electrochemistry data. In many regions of the cell, we saw the electrolyte was partially depleted, so ion transport became more difficult but not impossible. But in the three hotspots, the electrolyte largely ran out, so cycling became impossible.”
In addition to pinpointing the location of the hotspots where failure was occurring most rapidly, the synchrotron x-ray diffraction studies also revealed why failure was occurring there by providing the amount of NMC present at each position on the cathode. Regions with the worst failure typically had smaller amounts of NMC than the rest of the cell.
When less of the NMC cathode is present, that part of the battery charges and discharges more quickly and completely, causing the electrolyte to be consumed more rapidly and accelerating its eventual failure in these regions.
Reductions of less than 5% in the cathode amount can accelerate failure. Therefore, improving manufacturing processes to produce more uniform cathodes should lead to longer-lasting batteries.