With demand for renewable energy sources and electric vehicles continuing to rise, there is an increasing need for reliable, high-capacity energy storage. Lithium-ion batteries (LIBs) are ideal for the purpose, but understanding the microstructural features that influence the performance of multiple layers of air-sensitive LIB materials can be challenging. Donna Gosselin of JEOL USA looks at how recent developments in microscopy are helping LIB researchers and manufacturers to understand the materials involved, and so refine and improve this essential energy-storage technology.

LIBs have seen a large growth in popularity in recent years, thanks to their combination of longevity, small size, rechargeability and relatively low cost. The uptake of LIBs is currently driven by the need to store energy derived from renewable sources such as wind and solar, and the move to replace hydrocarbon-fuelled vehicles with their electric equivalents. In addition, LIBs are widely used in a range of consumer goods such as smartphones, laptops, tablets, digital cameras and portable power tools.
Although great strides have been made in LIB technology, advances continue apace, especially with regard to increasing energy density and cycle life, enhancing battery stability and safety through better control of interfaces, and reducing reliance upon scarce, high-cost materials and minerals.
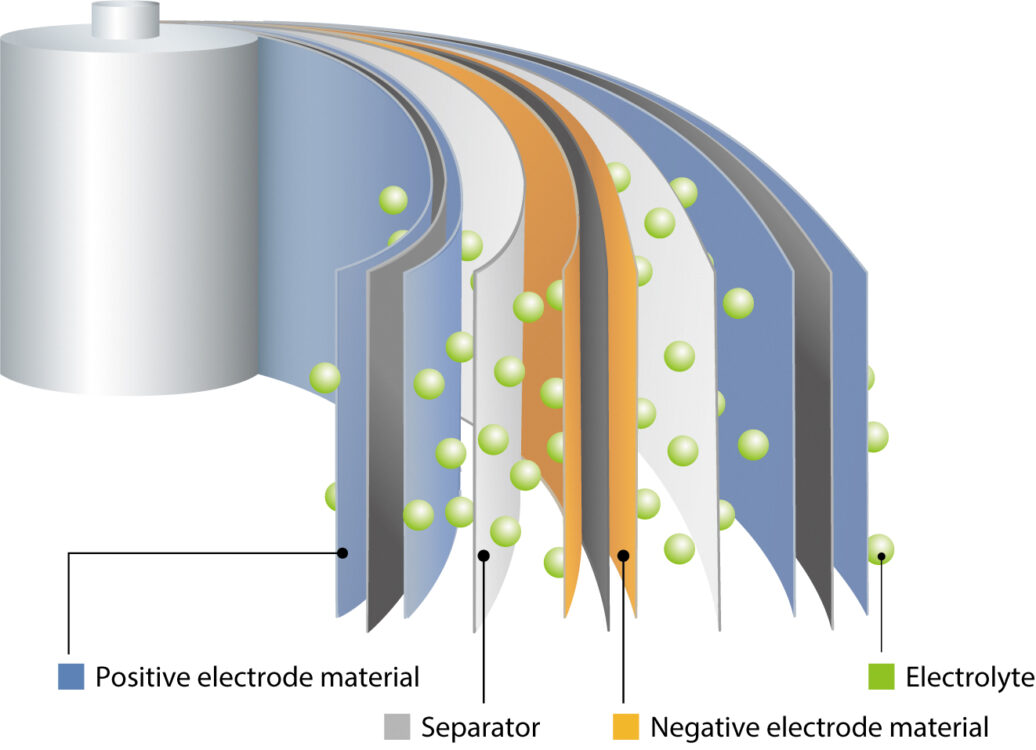
Researching, developing and optimising such new components requires an in-depth understanding of LIB chemistry and microstructure, but this is challenged by their structural complexity. For example, they can contain as many as 10 thin films fulfilling various purposes, including the cathode, anode, insulation, current collector and electrolyte. A variety of materials may be involved, including ceramics, metallic foils and polymers, which may take the form of powders, sheets or fluids.
Accordingly, analysis of LIB components depends upon an array of microscopic and spectroscopic methods. Analyses are typically carried out both before and after assembly, as well as after repeated charge/discharge operations. There are several challenges in LIB analysis, and new advances in analytical technology – specifically broad-beam ion milling and scanning electron microscopy (SEM) – can be used to tackle them.
Challenge 1: Reducing damage during cross-section preparation
Sample preparation, as the first step in any analysis, is crucial to obtaining reliable results. Traditional mechanical preparation of specimen surfaces for SEM can introduce artefacts such as scratches and embedded polishing media.
This obscures the original microstructure, compromising crystallographic information and making it difficult to obtain precise layer thickness measurements. Moreover, LIB materials that are brittle or porous can be badly degraded by such methods, and this is further complicated when LIB structures contain layers with differences in hardness or thermal expansion.
Broad-beam ion milling resolves these issues and has emerged as a preferred method for sensitive LIB samples. Using a shield plate ensures that only the exposed part of the sample gets milled away during cross-section preparation, reducing mechanical damage to the surface being revealed (Fig 2).
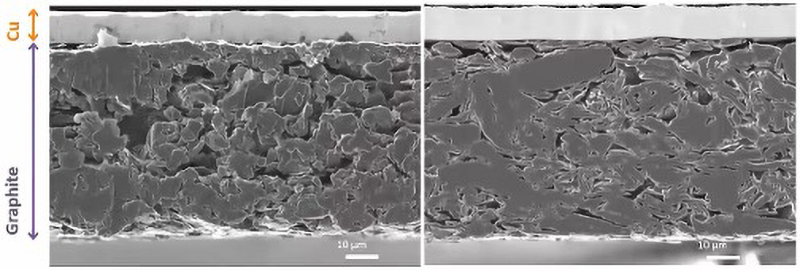
Cryogenic sample preparation systems are also available and they allow surfaces to be prepared at low temperatures. This offers benefits for temperature-sensitive samples (Fig 3), and inert-gas specimen exchange chambers enable air-sensitive lithium-containing specimens to be transferred from the ion miller to the SEM without exposing them to the atmosphere.
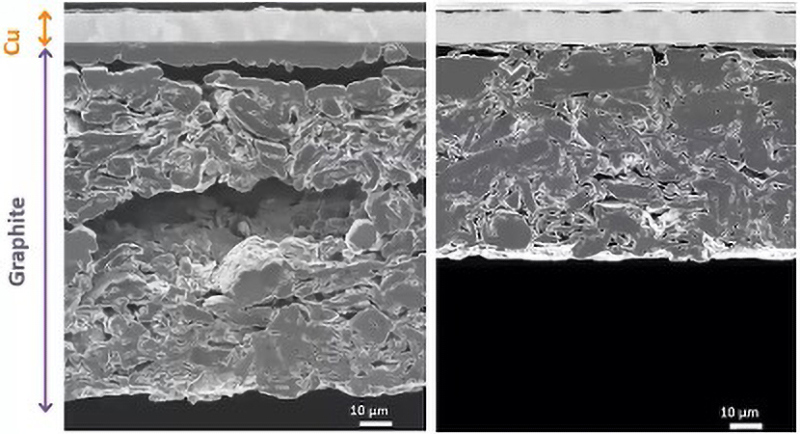
Challenge 2: Improving image resolution
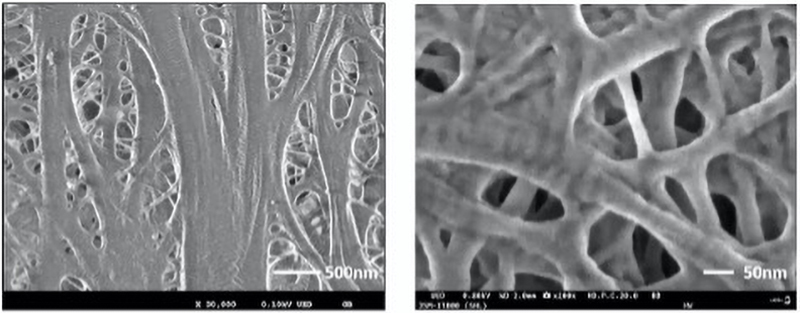
A critical aspect of any LIB analysis is understanding the three-dimensional microstructure of the individual layers, as well as of the interfaces between them. Avoiding surface damage is a particularly important factor for delicate LIB surfaces, and so there is an advantage to reducing the voltage to 2kV or below.
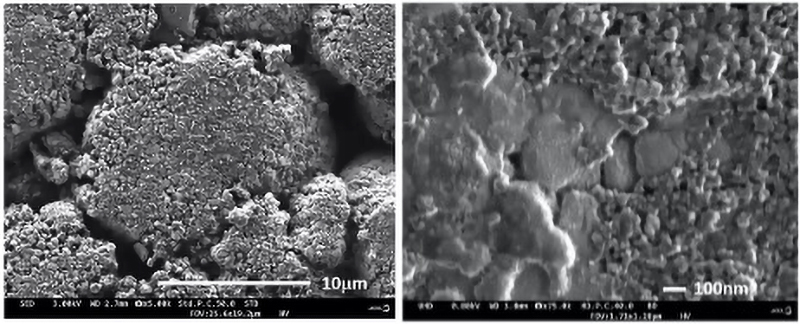
However, to avoid difficulties caused by generating very-low-energy electrons, instruments are now available that allow energy to be reduced after emission from the electron gun by applying a voltage bias to the specimen. With a further addition of an in-lens detector, images of LIB surfaces can be acquired with high resolution (Fig 4), and without causing damage to sensitive structures (Fig 5).
Challenge 3: Detecting light elements at low concentrations
Determining the identity, chemical state and distribution of elements in LIBs is vital for advancing the field. Using SEM fitted with an energy-dispersive spectroscopy (EDS) detector is a useful approach for mapping elemental compositions, but until recently it was not possible to detect the X-rays emitted by lithium.
This is because they are low-intensity, low-energy (54 eV for the Li-K line), and easily absorbed by the windows used on conventional EDS detectors. The development of windowless EDS detectors in the last 10 years has overcome this problem and opened interesting possibilities for detection of lithium in LIB materials (Fig 6).
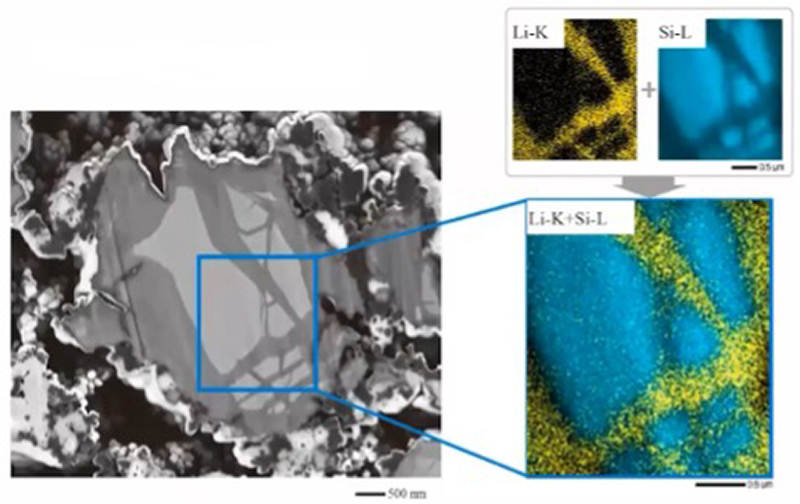
Another approach to elemental analysis of LIBs is electron microprobe analysis (EPMA), coupled with wavelength-dispersive spectroscopy (WDS). This requires smooth sections to be prepared but provides an order of magnitude more beam current than typical field-emission SEM, enabling detection of light elements at trace concentrations (Fig 7).
Challenge 4: Understanding the chemical environment
Beyond determining the identity of the elements present is the need for information on their chemical environment, in order to obtain a complete understanding of LIB microstructures and behaviour. In principle, X-ray emission spectroscopy can provide this information, but until recently, instruments were unable to provide X-rays with sufficiently low energy to access the necessary transitions.
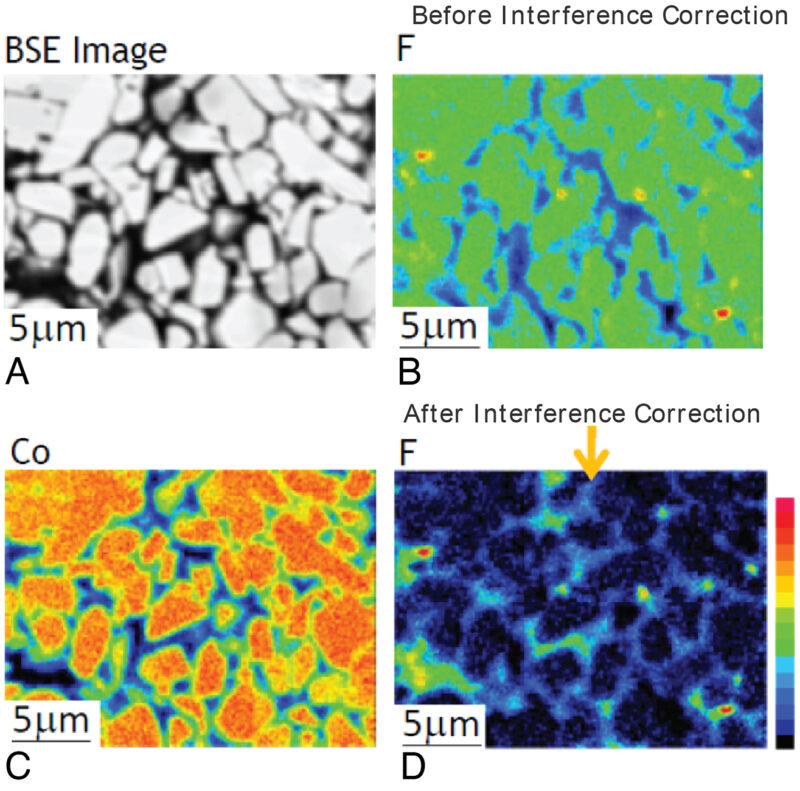
In 2016, a grating with variable line spacing was developed, which allows detection of characteristic ‘soft’ X-rays in the range 50-210 eV with high resolution and sensitivity. Importantly for LIBs, this soft X-ray emission spectroscopy (SXES) approach makes it possible to detect the Li-K emission directly, even at low single-digit wt% concentrations of lithium. This method can also be taken a step further: by coupling SXES to either a field-emission SEM or an EPMA, it is possible to point-map different chemical states (Fig 8).
Challenge 5: Detecting oxygen-bound Li
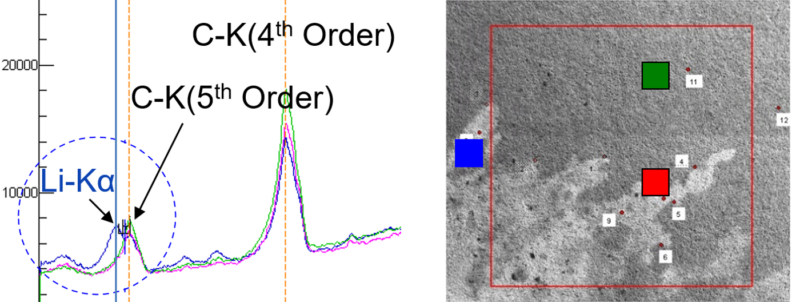
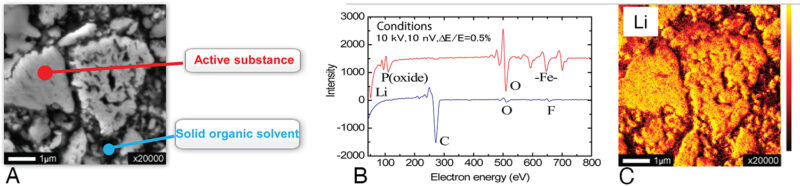
One limitation of X-ray spectroscopy is that when lithium is bonded to oxygen, the sole outer-shell electron is unavailable for demotion to the inner shell. Therefore, emission of X-rays from oxygen-bound lithium are minimal. However, Auger electrons can be emitted by virtue of a Coster–Kronig transition, allowing these electrons to be detected by Auger electron spectroscopy (AES) – and the chemical state of lithium determined – using an appropriate microprobe (Fig 9).
Conclusions
LIBs are an active field of study and making progress in the field depends upon access to a range of microscopy-enabled techniques for determining surface and cross-sectional morphology, elemental composition, and chemical environment. As described, advances in several of these techniques are proving particularly important for obtaining information about light elements (especially lithium) within complex layered structures and should be very useful for identifying new materials for use in LIBs, and further optimising their design and performance.
Donna Gosselin spent over 15 years as an analytical and research chemist in industry with extensive experience in R&D, product development and manufacturing across a broad range of industries. Donna is currently the SEM product manager for JEOL USA, Inc.