Until now dendritic growth has hampered improvements to a lithium battery’s energy density.
Now battery researchers Massachusetts Institute of technology (MIT) believe they have discovered how dendrites form, and more importantly how to prevent it.
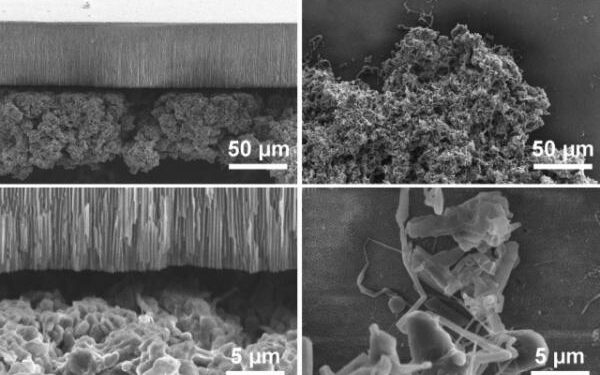
Researchers found deposits grow differently depending on the applied current, leading to the discovery there are two entirely different mechanisms at work.
While both forms of deposits are composed of lithium filaments, the way they grow depends on the applied current, the scientists found.
The first relatively easy to control clustered, ‘mossy’ deposits, form at low rates and grow from their roots, which can be blocked with a nanoporous ceramic separator.
The separators should be made of anodic aluminum oxide, which is 60 micrometers thick and has well-aligned, straight nanopores across its thickness, said the researchers.
The more ‘dangerous’ and harder to block dendritic projections grow only at their tips, and are more sparse and rapidly advancing, the research found.
However, the researchers said the average current associated with batteries was lower than that required to form the tip-growing deposits, meaning they would not form unless significant degradation of the electrolyte occurred.
Their findings were reported in the journal Energy and Environmental Science.
Martin Z. Bazant, a co-author of the paper, said: “While previous research has always lumped the two types of growth together under the blanket term ‘dendrites,’ the new work demonstrates the precise conditions for each distinct growth mode to occur, and how the mossy type can be relatively easily controlled.
“It’s a big discovery, because it answers the question of why you sometimes have better cycling performance when you use ceramic separators,” said the paper’s co-author Peng Bai.
The finding came after the team developed a laboratory setup, a glass capillary cell, that allowed them to see the transition from one kind of growth to the other.
The paper was also authored by Ju Li and Fikile Brushett.