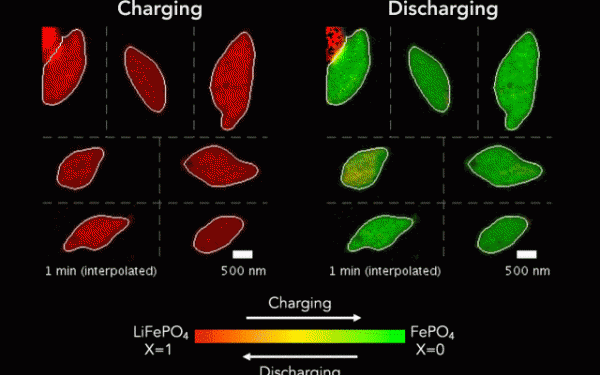
Scientists may have discovered how to design better battery and management systems after observing how lithium-ions move at the nanoscale.
The team used X-rays and cutting-edge microscopes at Lawrence Berkeley National Laboratory’s Advanced Light Source to look at the particles as they were charged and discharged.
The researchers discovered delithiation is significantly less uniform than lithiation, but became more uniform with faster charging.
The results were published in the journal Science.
A battery’s performance is hampered when areas of the electrode take on more ions, and others fewer. This leads to the crystal lattice becoming overburdened with ions and developing tiny fractures.
The team was led by William Chueh, an assistant professor of materials science and engineering at Stanford and a faculty scientist at the Department of Energy’s SLAC National Accelerator Laboratory.
Yiyang Li, a doctoral candidate in Chueh’s laboratory and co-lead author of the paper, said: “Lithiation and delithiation should be homogenous and uniform.
“In reality, however, they’re very non-uniform. In our better understanding of the process, this paper lays out a path toward suppressing the phenomenon.”
The team made a battery, in collaboration with Hummingbird Scientific, of two transparent silicon nitride windows and a electrode made of a single layer of lithium iron phosphate nanoparticles.
Picture: Jongwoo Lim, Yiyang Li, and William Chueh of Stanford and SLAC National Accelerator Laboratory and David Shapiro of Lawrence Berkeley National Laboratory stand in front of the X-ray microscope at the Advanced Light Source. (Image credit: Paul Mueller/Lawrence Berkeley National Laboratory)