Scientists at the Massachusetts Institute of Technology (MIT) have developed a safer, cheaper and more scalable sealed lithium-air battery.
The new battery employs conventional electrochemical reactions between lithium and oxygen during charging and discharging, but retains the oxygen in a solid state.
Current lithium-air technology draws in gaseous oxygen from outside the battery to drive a chemical reaction before releasing it again.
Findings for the new battery concept, called a ‘nanolithia cathode battery’, was published in the journal Nature Energy.
The paper was written by Ju Li, the Battelle Energy Alliance Professor of Nuclear Science and Engineering at MIT, postdoctoral Zhi Zhu, and five others at MIT, Argonne National Laboratory, and Peking University in China.
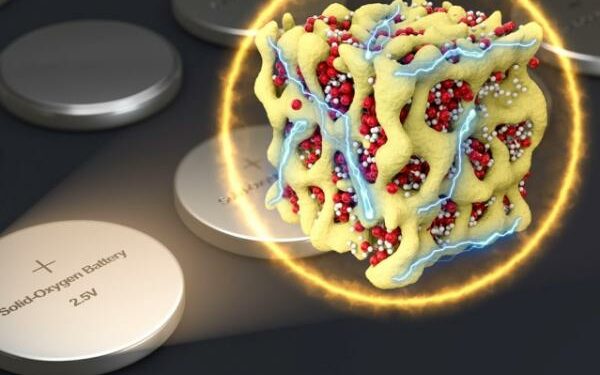
The battery’s secret lies in creating minuscule particles, at the nanometer scale, that contain both the lithium and the oxygen in the form of a glass (formed by Li2O, Li2O2, and LiO2), confined tightly within a stabalising matrix of cobalt oxide, says the paper.
Li calls these particles nanolithia (hence the name). In this form, the movements between Li2O, Li2O2, and LiO2 can take place entirely inside the solid material.
This reduces the voltage loss from 1.2 volts to 0.24 volts, so only 8% of the electrical energy is turned to heat, claim the scientists.
Li said: “One of the shortcomings of lithium-air batteries is the mismatch between the voltages involved in charging and discharging the batteries.
“The batteries’ output voltage is more than 1.2 volts lower than the voltage used to charge them, which represents a significant power loss incurred in each charging cycle.
“You waste 30% of the electrical energy as heat in charging. It can actually burn if you charge it too fast.”
The new battery showed less than a 2% capacity fade when put through 120 full cycles in laboratory tests, the paper stated.
Li believes the new battery system is more scalable, cheaper, and safer than traditional lithium-air batteries.
Image: In a new concept for battery cathodes, nanometer-scale particles made of lithium and oxygen compounds (depicted in red and white) are embedded in a sponge-like lattice (yellow) of cobalt oxide, which keeps them stable.