The 40th International Battery Seminar and Exhibit was the biggest yet, extending its welcome to well over 2,000 delegates and more than 200 exhibits. Keynote speaker Prof. Jeff Dahn, of Dalhousie University in Nova Scotia, Canada, shared the results of laboratory testing designed to come up with batteries that last exceptional lifetimes. He reckons 50 years is feasible. BEST was there to hear more.
Jeff Dahn’s group published a paper in 2019 showing “some pretty excellent testing results” for NMC532 single crystal cells, in which they claimed they could run a vehicle for more than a million miles. This chemistry came to be called the “million mile battery” in the media.
Three years of subsequent and continuous testing produced even better results, he said in his keynote presentation to IBSE.
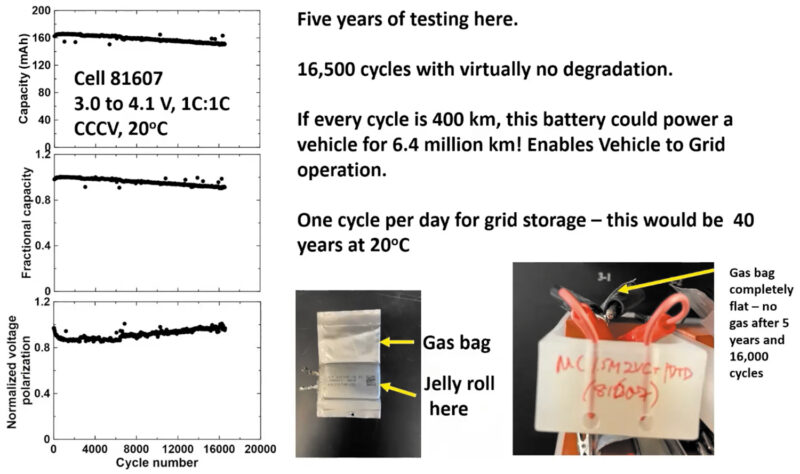
The single crystal NMC532/graphite cells ran at 20°C , at 3‑4.1V; nominal 1C: 1C cycling with a C/20, C/2, 1C, 2C, 3C rate map every 100 cycles. One cell has run for more than five years. It has got 16,500 cycles, virtually no degradation, has over 90% capacity remaining and no measurable increase in the internal resistance of the cell.
A 6.4 million kilometre battery
“Just for your interest, if every cycle is 400km, this battery could power a vehicle for 6.4 million km. It’s way more than you would ever drive. So a cell like this enables vehicle-to-grid, where you have absolutely no worries about having your vehicle plugged in and doing several charge discharges a day to support the grid.
“If you use this cell and grid storage and maintain the battery at 20°C, you’d get 40 years or so of operation.”
He said an attached gas bag showed absolutely no gas generation over five years, indicative of very little electrolyte electrode reactions.”
Elevating the temperature
Testing at room temperature takes “way too long”, so his team tests and tries to improve things at elevated temperatures. Tests were done using the same chemistry at 55°C, with cells running up to 4.1, 4.2, 4.3 and 4.4V. After 4,000 cycles at 4.1V, things were “pretty decent”, he said.
But voltage polarisation (the difference between the average charge and discharge voltages) increased with cycle number, due to reactions between the charged electro materials and the electrolyte at elevated temperature. The higher the upper cut-off voltage, the worse it gets, he said. “And you can see at 4.4V, the cells are going to eventually roll over here and fail, much before the other cells.”
He showed results of a cell operating at an upper cut-off of 3.8V, and where the electrolyte degradation was “absolutely minimal” by comparison.
NMC chemistries charged at the higher 3.9-4.1V showed increasing advantage over LFP, he said.
“Things last a really long time for these low voltage NMC cells with LiFSI. But when you use this DMOHC, it’s a year. A year is 8,700 hours. It’s a year at 85°C with about 10% degradation,” he said, noting that Tesla has patented use of DMOHC, an electrolyte solvent.
“Incredible” capacity retention
He showed a nickel 83 cell that had been operating at 70°C for over a year. Voltage polarisation had not increased at all and there was no internal impedance growth. “The capacity retention is incredible. You know, I had the benefit of watching this thing happen over the year. You know, every week you go and look at the charger, you just fall off your chair,” he said. “Seriously, this is crazy.”
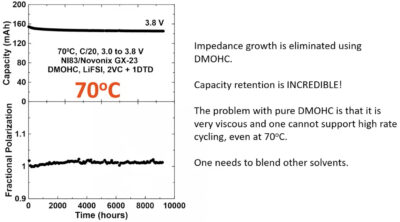
He believes that if a lithium-ion battery can run in a year’s testing at 85°C, at room temperature it will be around 50 years.
The cells have a lithium reservoir that can be used to extend the lifetime by a further factor of at least two.
The lifetime of the cells is going to be much longer, but what matters most is the cost of energy stored over the lifetime, especially for grid storage. It costs more upfront but lasts much longer. Cells can also be made from cobalt-free materials like NMC640.
They are suited to grid energy storage, battery pack swapping, two-wheel e-mobility vehicles that are air-cooled in hot climates and presently last just a couple of years, Evs with V2G capability and still have a second life.
On the issue of safety Dahn stated that at 3.8V NMC is “pretty darn benign… NMC gets worse the higher the voltage goes.”
For lithium batteries in grid storage, it costs a lot of time and money to replace installed systems so, he believes, you should view an installation as a publicly-owned piece of infrastructure – like a bridge – and consider the longevity.
Lithium metal is a holy grail that gives a headache

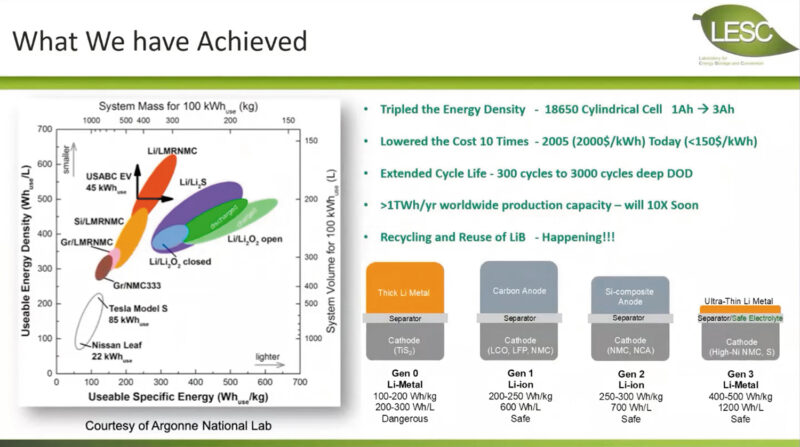
Prof Meng said much has been achieved already in terms of research:
- The 18650 cylindrical cell has tripled energy density (from 1Ah to 3Ah)
- Costs have lowered from $2,000/kWh in 2005 to $150/kWh
- Life cycle extended
- Worldwide production capacity of 1TWh/year will soon increase 10-fold
- Recycling of lithium-ion batteries is already happening.
Reducing copper and using pure lithium foil makes a major impact on energy density, she said. There is still some capacity degradation at the end of life, but lithium metal batteries are here to stay.
“I want to show solid-state batteries as a platform technology where we can do some very interesting material science. The first major breakthrough in the solid-state batteries is the making of that 30-micron thick membrane with inorganic materials and potentially the help of polymers. It actually is a reality today we can make solid-state electrolyte with less than 30-micrometre thickness, and that immediately brings us in energy density.”
She said the goal in solid-state is to achieve several things. One is the volumetric energy density. Getting over 1,000 in solid-state cannot be achieved, she said, but it would be very good if they can reach 801 hour per litre.
Her team was able to produce freestanding LiPON less than four micrometres free standing. The electrolyte interface remains “amazingly stable after many, many, many cycles”.
She believes solid-state will start with niche markets where internet-of-things items, mobile phones or watches could be the first application, enabling the maximum collection of data to learn fast about manufacturing.
Lithium metal is her biggest headache as it is “very tricky to handle in solid-state”. High pressure during cycling always causes it to short circuit, she said, though produces no fires.
Cost of solid electrolyte also needs to come down to make solid-state batteries feasible, she said.












