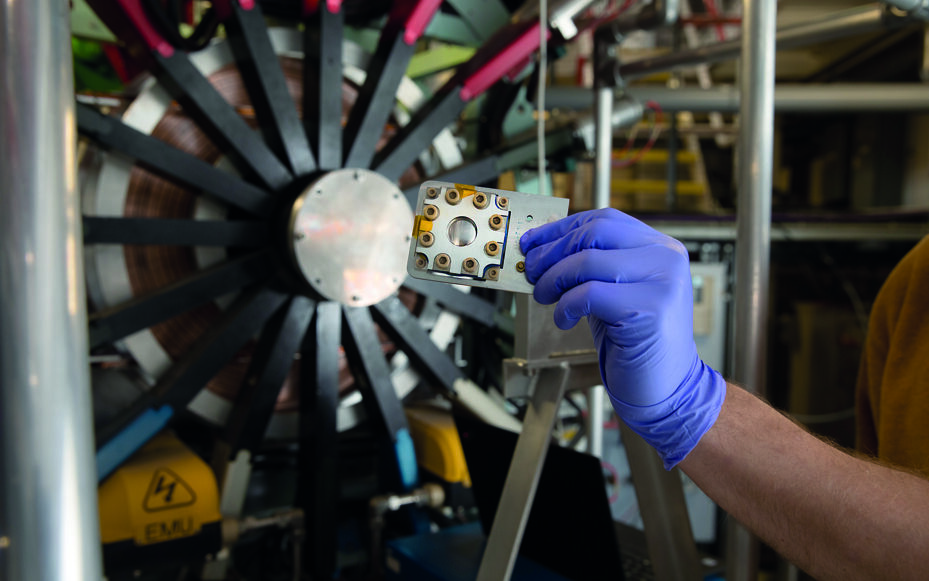
Expanding our understanding of battery materials, how to optimise them further, and how to make new ones based on abundant raw materials will be key to aiding the world’s transition to net-zero carbon emissions. Doctor Peter Baker— instrument scientist at the ISIS Neutron and Muon Source, and co-investigator on the Faraday Institution FutureCat project— explains how muons are advancing our knowledge in two-millionths of a second bursts.
Now more than ever we need to change a wide range of human activities and support significant innovation— at the forefront of . . .
to continue reading this article...
Sign up to any Premium subscription to continue reading
To read this article, and get access to all the Premium content on bestmag.co.uk, sign up for a Premium subscription.
view subscription optionsAlready Subscribed? Log In