A discovery by chemists at a North American university could change how non-aqueous metal-oxygen batteries are developed.
Researchers from the Waterloo Institute for Nanotechnology, led by Professor Linda Nazar, uncovered a key reaction in sodium-air batteries – which could have implications for other chemistries.
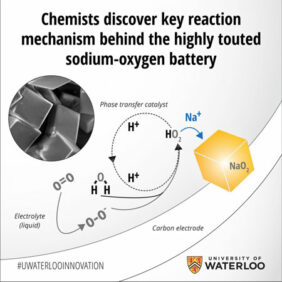
By isolating the proton phase transfer catalyst in the battery’s discharge and recharge reactions, the team was able to boost capacity, and achieve a near-perfect recharge of the cell.
The findings on the critical role and mechanism of phase-transfer catalysis in Na–O2 batteries were published on Nature magazine’s website.
Researchers found the catalysis is solely responsible for the growth and dissolution of micrometre-sized cubic NaO2 crystals and for the reversible cell capacity.
The findings by Nazar, who holds the Canada Research Chair in Solid State Energy Materials, could lead to extending lithium–air cells, although this will require an additional redox mediator to charge the cell efficiently.