Power management innovator Chris Hale looks into the viability of second-life for lithium-ion batteries once their use in electric vehicles draws to a close. Does the case for second life stack up or will it disappear into the black hole of good ideas?

BEST has previously reported on some of the issues surrounding the recycling of lithium-ion battery packs, particularly as there is a growing concern with the sheer volumes that are entering service now, not to mention the volumes expected in the years to come. Even still, there are questions as to how soon and how many lithium-ion recycling plants will be brought online in the coming years and until they do, does it not make sense to stretch out the useful life of existing battery packs? The ability to push back the time before batteries are sent for disposal or recycling should surely not just simply be a question of economics.
There are environmental and humanitarian aspects that should be considered, such as the mining of toxic materials. Cobalt, for example, is a heavy metal where mining has been particularly contentious, especially with the exploitation of child miners in countries such as the Democratic Republic of Congo, where 60% of the world’s cobalt is mined, as well as Zambia and Cuba. Lung disease and heart failure have all been attributed to cobalt, and with any batteries disposed of in landfill sites, there is always the risk of contamination seeping into the soil and groundwater. In 2017, the world’s battery makers used 41,000 tonnes of cobalt (a third of total production). By 2025, this is expected to increase to 117,000 tonnes.
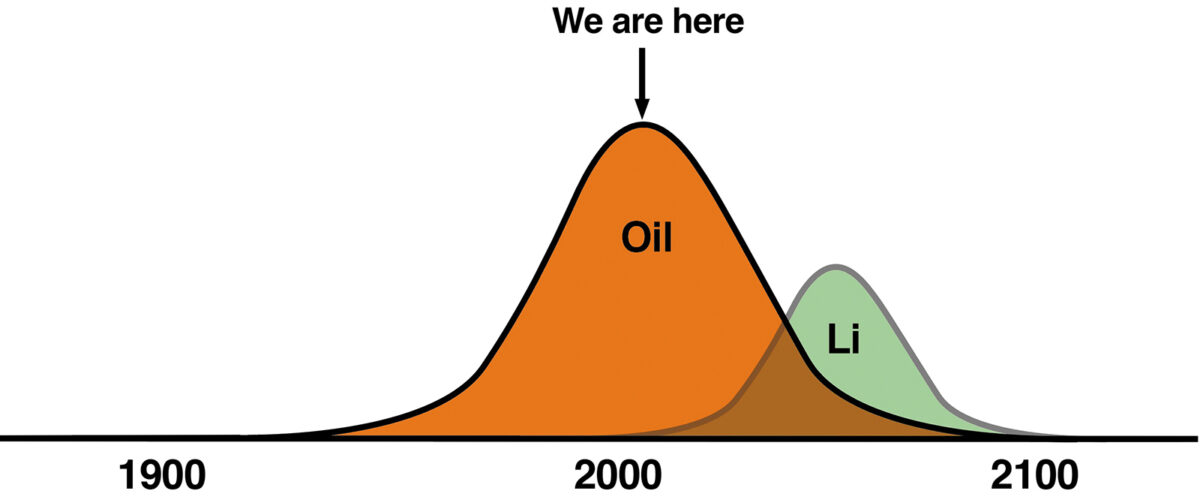
Then there’s the supply of lithium, a finite resource with limited global lithium reserves. According to researchers at the German university LUT-Ausgsberg, various models were examined to determine how much lithium remains on Earth with estimates from 30-95 million tonnes. For these figures, projections on when the supply would run dry depended on a number of scenarios but, in short, gave timeframes of 2040–2100. We plan to replace oil, a finite resource, with batteries predominantly using lithium— another finite resource. Especially when the transition from fossil fuels will result in a significant dependence on electric vehicle (E) batteries.
If we consider the waste from spent lithium-ion batteries for EVs sold globally in 2017 alone, this could amount to 250,000 tonnes when they reach the scrap heap in 2027. By 2040 this figure could be close to four million tonnes.
Ultimately, our choices are going to boil down to burying it (landfill) or recycling it. Bury and the resource is lost, recycle and some— perhaps not all— can be reclaimed. Bare in mind the projections above included provision for recycling.
So what is the argument for extending the useful life of an EV battery system by re-purposing in a second-life application? By second life we are extending the period before the battery has to go to recycling, hopefully enabling catch-up of lithium-ion recycling plants to recover more of the raw materials. But what of a counter-argument for supply? If there is projected to be a significant increase in EVs then surely it makes sense to recover the raw materials as soon as possible to feed back into the supply chain. This will all drive the economics and enforces the debate on whether second life is economically viable.
To put this further into perspective, the material in a Tesla battery, for example, is worth around $1,500— but the market value of a new Tesla battery pack is between $10,000 and $15,000, so most of the cost lies within the manufacturing processes. If a battery is re-sold into a second-life application it would typically have a value of 60% of a new battery, so the labour and additional manufacturing processes need to be kept low.
In a nutshell, when a lithium-ion EV battery comes to the end of its life, it still retains around 80% of its charge. The remaining charge is still good enough, however, to serve a variety of different applications, such as energy storage for one, and as such is a key focus for many re-purposing companies.
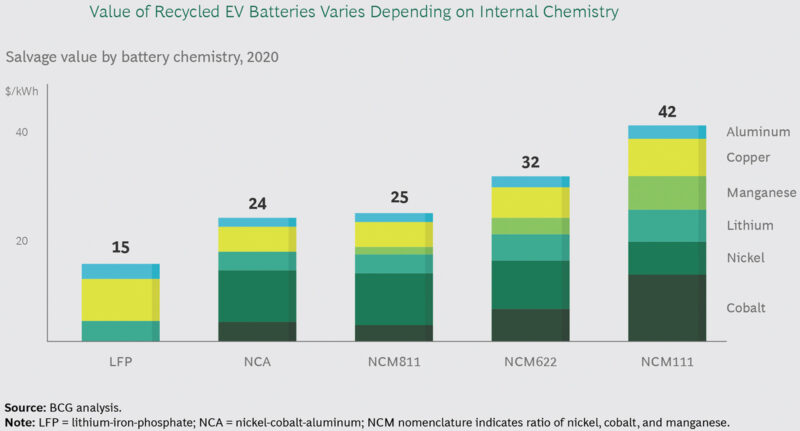
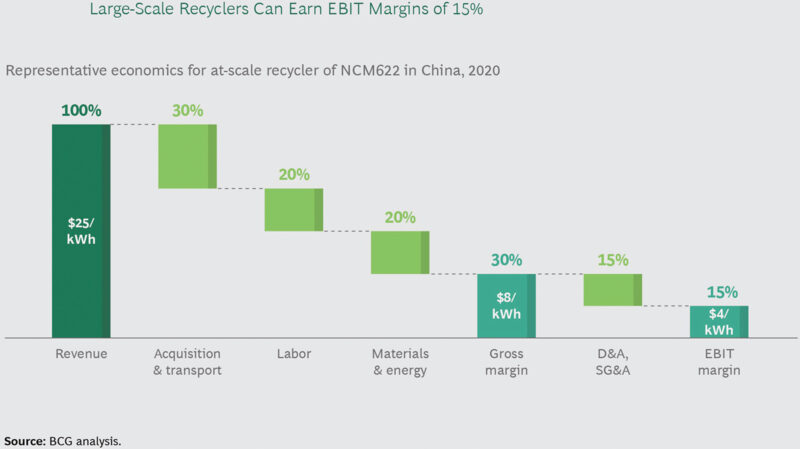
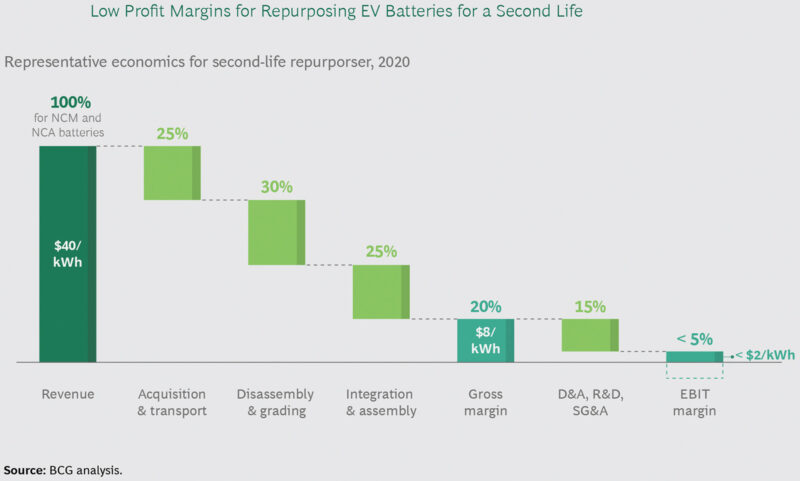
This being said, according to Boston Consulting Group (BCG) analysis, it is EV recycling at a scale that can be demonstrated as being the more attractive option economically, when taking into account the value of the materials (Fig 1) and the potential margins that may be achieved (Fig 2). This is in contrast to the margins expected for reuse (Fig 3) and does pose the question of where and why the reuse economics are more challenging and what can be done to improve them.
BCG outline three possible waste streams:
- In recycling, a specialised company recovers valuable metals— including cobalt, manganese, nickel, and lithium— from the battery cells. It then sells these materials for use in a future battery manufacturing process.
- In a second-life application, a specialised company repurposes the battery cells (or modules) for a new use, without dismantling them, often in combination with a new set of power electronics, software and enclosures.
- If placed into a waste stream, the battery may enter a landfill or another disposal facility with limited recovery of any of its remaining value. Increasingly, regulations are mandating that lithium-ion batteries enter the circular economy rather than being discarded.
According to the BCG, estimates for demand in batteries for the stationary energy storage (SES) market alone will likely reach 120GWh annually by 2030, so there is plenty of potential demand for a second-life battery system. However, stakeholders must overcome the current economic obstacles to sustaining second-life battery applications.
In this regard, original equipment manufacturers (OEMs) have several advantages over SES providers. Not only are they likely to have access to a high volume of end-of-first-life batteries, but they can also apply their in-house design and integration capabilities to the task of repurposing old batteries. Not only that, they have control over the battery management system (BMS) and any historical data that aids with the grading process.
The production process for repurposing a battery, which includes disassembly to either module or cell level, is complex. The cells or modules must be tested, graded, and matched; new enclosures must be provided along with a new BMS or software to suit the new application, and the repurposed battery must be reassembled and tested before it can be resold.
Disassembling, grading, and reassembling batteries is a time-intensive, largely manual process, and it is further complicated by limited knowledge about how the battery had been used and its current cell-level performance. The grading process is one area where research is focused on streamlining to reduce the time from days to minutes.
An additional challenge comes with the reduced value of second-life batteries when compared with those of new, especially when taking into account that the price of new batteries continues to fall.
Reliability and safety are also key, batteries nearing the end of life are precipitating internal degradation mechanisms that could result in sudden and potentially catastrophic failure, so state-of-health (SoH) and state-of-failure (SoF) predictions are essential.
All things considered, the analysis shows that repurposing EV batteries for second-life applications is currently economically challenging, in Europe and the US, on a standalone basis, as the potential profit is too low to make the effort worthwhile (Fig 3).
Certainly, it looks tight, but the challenge here is more about how to improve the disassembly and grading plus the integration and assembly processes. If these can be made more economical then surely the earnings before interest and tax margins would fall in line.
So, what is being done to address this? In effect, disassembly can be improved through greater re-manufacture consideration during pack design. If OEMs are persuaded to consider disassembly potential when designing their packs this may have a significant impact. Certainly, processes such as wire-bonding to the top and side lip of a cell make disassembly at the cell level easier than traditional approaches of top and bottom welded plates. That’s one approach at the cell level, however many second-life applications simply disassemble the pack down to the module level, grading the independent modules rather than cells. The grading process can be fairly time-consuming and costly, typically taking many hours and requiring expensive charge/discharge systems.
So, what can be done here? In the Winter 2021 issue of BEST, professor David Greenwood, CEO at WMG University of Warwick, highlighted potential solutions to the grading issues. The tests they developed characterised the response of the battery to AC signals over a range of frequencies, and they used this to develop a ‘fingerprint’ for the battery that can be compared with the known responses of batteries at different stages of ageing.
This essentially uses a process of electrochemical impedance spectroscopy (EIS), a technique that is becoming more popular within BMS design to ascertain the SoH of a cell, rather than the common method of full charge/discharge cycles to evaluate SoH. There are in fact a number of dedicated integrated circuit (IC) devices currently available, such as the Analog Devices AD5941 High Precision Impedance and Electrochemical Front End (others are available), which help to incorporate SoH features in a cutting-edge BMS.
The EIS measurement approach consists in applying a sinusoidal current or voltage of a certain amplitude and frequency to the battery and measuring the phase shift and amplitude of the output voltage or current.
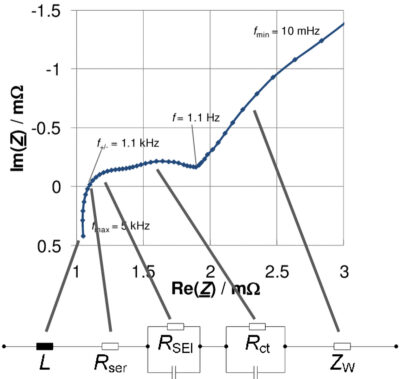
The impedance spectra presented in Fig 4 are composed of five distinct sections relating to different aspects of the cell. At very high frequencies, the impedance spectra show an inductive behaviour (L); the value of the ohmic resistance Rser of the battery is found at the intersection of the impedance spectra with the real axis (Im(Z) = 0); the third section is represented by a small curve, which is related to the solid electrolyte interface (SEI) layer; the larger curve (fourth section) is associated with the charge-transfer process and the double-layer capacitance; the fifth section, located at small frequencies, corresponds to the diffusion process (Warburg coefficient σ).
The key advantage of this approach is that a battery can be measured, analysed and characterised without knowing any of its prior history.
Data gathering by the BMS, monitoring or tracking of ageing, and SoH are all very well and good, but tend to be proprietary to the battery manufacturers and not generally accessible to companies repurposing modules for second-life applications. The EIS approach is therefore a useful tool for battery grading purposes and generally fairly straightforward to implement. Fig 5 shows an example of the response to EIS testing identifying the characteristic differences between a good battery and one with faded capacity.
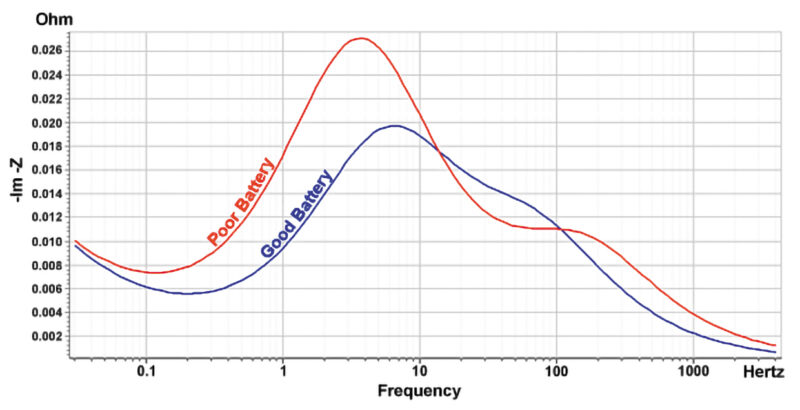
Batteries with faded capacity suffer from low charge transfer and slow active lithium-ion diffusion.
In addition, the EIS analysis is also a good tool for functional safety and SoF detection, with tell-tale markers indicating the approach of a sudden demise.
As seen in the diagrams below, accelerated ageing using a process of over-charge cycling has a noticeable and detectable characteristic change in the measured readings. In brief, the four diagrams of Fig 6 demonstrate where tell-tale indicators project the imminent demise of the cell. The readings are taken at 50% SoC for the Ohmic resistance, RO, the SEI resistance, RSEI, the charge transfer resistance, Rct and the Warburg coefficient σ.
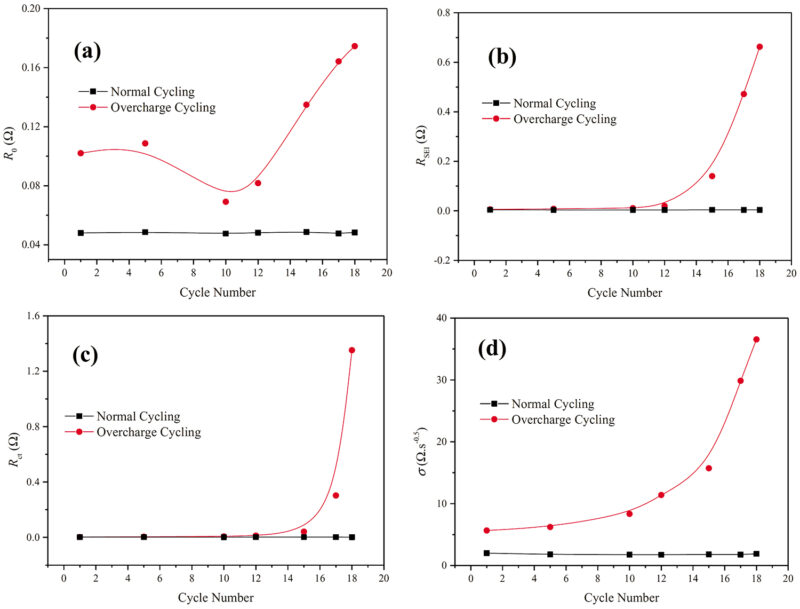
In general, RO had a clear trend of increasing with cycle number, which was similar to that of tests carried out at 0% SoC and could serve as an early warning signal for the failure of the cell. Another effective indicator associated with cell failure is the Warburg coefficient, as shown in Fig 6(d) which showed a steep rise in the cycles just prior to the failure on cycle 18. The resistance of SEI (RSEI) represented a sudden increase after the 15th cycle (right before the failure), which can also be used as an indicator to predict failure. Additionally, Rct in Fig 6 (c) showed similar trends with cycle numbers, indicating a pending demise.
At the end of the day, it is the Warburg coefficient (σ) that may best be used as a very effective measure to predict the failure of the cell, potentially avoiding incidents of thermal runaway or catastrophic failure. Overall though, it is clear that RO, RSEI, and the Warburg coefficient, are all associated with cell failure and can each be used as an early warning of imminent failure.
In support, it is also worth making a note of the more traditional method for determining an ageing cell using an approach of current impulse detection. This approach provides a much less accurate state of pending failure but can be used to support SoH and end-of-life determination based on increased resistance as demonstrated in Fig 7.
If we can demonstrate grading approaches that do not require lengthy discharge/charge cycles, where does this leave us in the debate over the economics of reuse vs recycling? The process of EIS is a great tool for grading cells and batteries, potentially reducing the time and resource requirements attributing towards the grading costs, but also demonstrates the requirement for advanced BMSs to be incorporated in order to continue SoF monitoring and mitigate the potential of catastrophic failure. The disassembly costs to the module level could be tackled by OEMs taking on the mantle of a better design for re-manufacture, which then just leaves consideration for the integration and assembly costs.
If the existing BMSs can’t be re-purposed for the new application, then replacement BMSs will add to the cost. But, as mentioned previously, when recycling becomes more widespread and effective at recovering raw materials, the supply and demand of these raw materials in the growing EV market may change the economics away from second life. But I don’t see that we are there yet and, for now, the second-life focus should still remain a viable option.