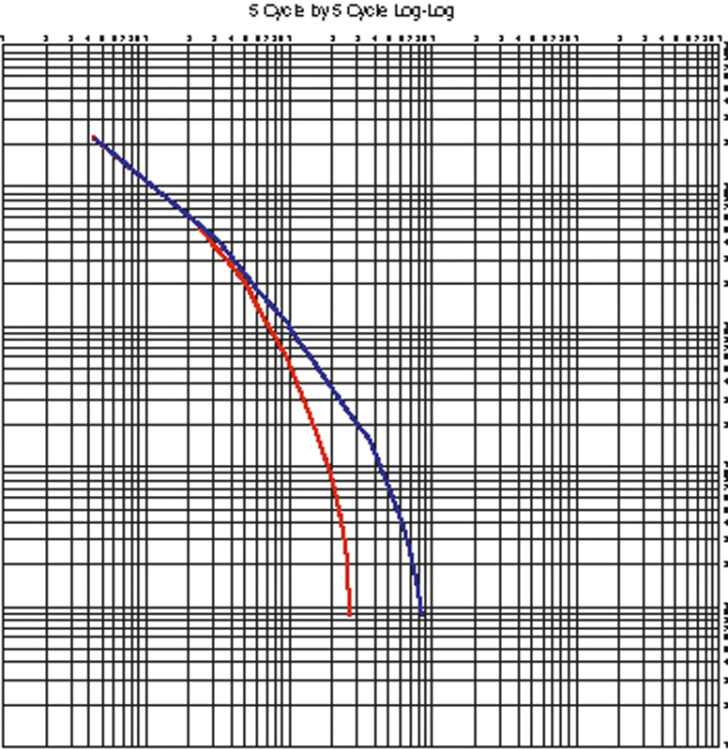
George Mayer explains the need for a Peukert curve, when customers' demand profiles don't quite fit what the battery maker had in mind.
Although I really wanted to tear directly into some pithy and interesting technical issue like pasting and curing in the summer issue, it is more practical to start with the Battery User (the Customer). Nothing happens without a sale.
We all know that a variety of battery sizes and designs are available from multiple vendors worldwide. Although the boxes may be physically identical (thanks to BCI, SAE, DIN, JIS and other standards . . .
to continue reading this article...
Sign up to any Premium subscription to continue reading
To read this article, and get access to all the Premium content on bestmag.co.uk, sign up for a Premium subscription.
view subscription optionsAlready Subscribed? Log In