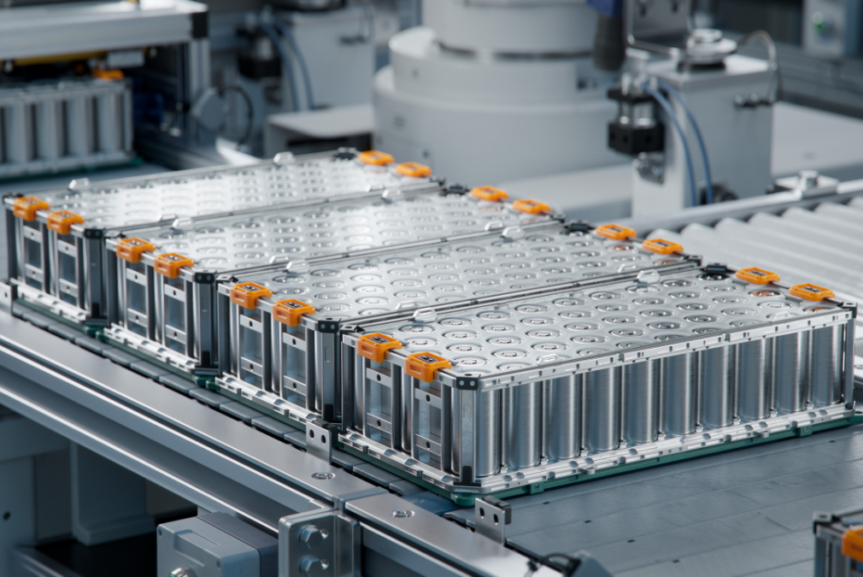
BESTmag technical editor Dr Mike McDonagh is back in the lab with UK Powertech's Mark Rigby and Digatron equipment— testing for formation inefficiency and energy losses from corroded connectors.
The latest in a series of tests conducted in the Manchester-based laboratory in the UK is aimed at showing how the energy losses in lead-acid battery formation processes can manifest themselves without necessarily registering as a higher energy usage on conventional formation equipment.
For instance, a high resistance connection should . . .
to continue reading this article...
Sign up to any Premium subscription to continue reading
To read this article, and get access to all the Premium content on bestmag.co.uk, sign up for a Premium subscription.
view subscription optionsAlready Subscribed? Log In