Researchers from the US have taken the first images of the formation of solid-electrolyte interphase (SEI) and with it potentially boosting the performance of lithium-ion batteries.
The results suggest the right electrolyte could minimise the swelling and improve the battery’s performance, paving the way for researchers to tweak and improve battery design.
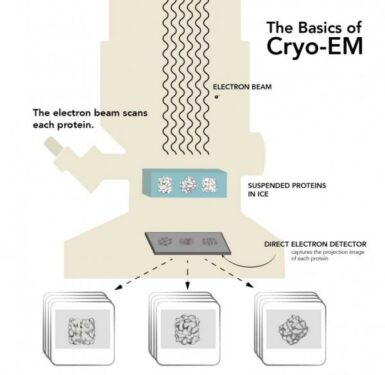
The team from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University used cryogenic electron microscopy (cryo-EM) to take high-res images of the SEI in its “natural plump, squishy state”.
The images helped the scientists better understand why the battery’s electrolyte corrodes the surface of the lithium metal anode, which creates the SEI.
It is believed formation of SEI is inevitable, but research around stabilising and controlling the thin layer of gunk aims to maximise a battery’s performance.
The team described their work in a paper published in the peer-reviewed journal Science.
Zewen Zhang, a Stanford PhD student who led the experiments with SLAC and Stanford professors Yi Cui and Wah Chiu, said: “We wanted to prove that we could image the interface at these previously inaccessible scales and see the pristine, native state of these materials as they are in batteries.
“We find this swelling is almost universal. Its effects have not been widely appreciated by the battery research community before, but we found that it has a significant impact on battery performance.”
Cryo-EM technology
The research is the latest in a series of results over the past five years that show cryo-EM’s use in energy research.
Cryo-EM is a form of electron microscopy, which uses electrons rather than light to observe the world of the very small, and was originally developed as a tool for biology.
Cui teamed up with Chiu to explore whether cryo-EM could be as useful a tool for studying energy-related materials.
They looked at SEI layers on a battery electrode and published the first atomic-scale images of this layer in 2017.
To make atomic-scale images of this layer in its native environment, the researchers inserted a metal grid into a working coin cell battery.
When they removed it, thin films of electrolyte clung to tiny circular holes within the grid, held in place by surface tension, and SEI layers had formed on tiny lithium wires in those holes.
Researchers blotted away excess liquid before plunging the grid into liquid nitrogen to freeze the films into a glassy state for examination with cryo-EM.
This yielded the first detailed images of the SEI layer in its natural swollen state.
Zhang said: “Right now that connection between SEI swelling behavior and performance applies to lithium metal anodes, but we think it should apply as a general rule to other metallic anodes, as well.”