Sponsored article
The lead-acid battery is ideal for energy storage applications due to its operational safety and low cost. However, the cycling performance of the positive electrode is significantly compromised by fast capacity decay caused by the softening and shedding of positive active material (PAM). This article from Eternity Technologies compares the influence of high- and low-temperature curing algorithms on the 70% DoD cycle life of industrial valve regulated gel batteries.
In the field of lead-acid batteries, the curing process has a profound effect on battery performance. Manufacturers universally adopt two curing methods: low-temperature curing (LTC) that creates tribasic (3BS) crystals and high-temperature curing (HTC) that creates tetrabasic (4BS) crystals.
Batteries with 3BS cured plates exhibit good high rate discharge performance and are ideal for SLI use. However, they do not perform so well in deep-discharge applications.
Curing involves the oxidation of free lead within the active material of a lead-acid pasted plate. This process, facilitated in a humidity and temperature controlled chamber, also creates a binding corrosion layer between the grid and the active material, Fig 1.
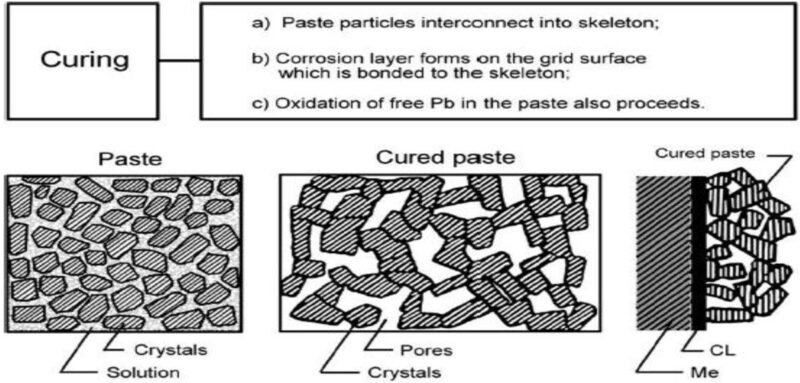
The curing reaction also establishes a foundational skeleton layer. The initial skeleton formation is achieved through controlled curing temperatures and humidity levels. The curing conditions are also dependent on the moisture level and free lead content of the paste mix.
Curing chamber performance
Chamber preparation and maintenance are critical for consistent curing performance. Aerosol-type curing chambers offer cost-effectiveness and yield cured plate surfaces with minimal cracking. However, they tend to exhibit higher inconsistency in process control, often due to water quality and pressure variation.
Conversely, steam-supply chambers require higher investment and maintenance levels but provide consistent process control, lending themselves to continuous production and higher temperature curing profiles. They maintain the required humidity levels to prevent sudden moisture loss from the plates. For our curing temperature study, we opted for steam-supply curing chambers.
Oxide production
We had opted for ball mill oxide for plate manufacturing due to its high acid absorption properties. Attaining a favourable exothermic reaction in the curing process relied on the initial residual lead content of the oxide mill. The standard specification for residual lead ranged from 28–32%, regardless of the oxide source. Lower residual lead content, below 15% – resulting from ageing, paste mixing, or pasting – was inadequate for an effective curing reaction and could lead to poor bonding. We maintained a residual lead range of 20–25% after the pasting process.
Factors affecting paste mixing process
Paste mixing (Fig 2) plays a pivotal role in the curing process, ensuring the preservation of maximum residual lead levels and moisture content. Inadequate paste mixing methodologies can undermine curing reaction performance.

Maintaining peak temperature during paste mixing was crucial, as it determined the formation of 3BS and 4BS crystals during curing. To achieve this, paste mixing required water cooling and air cooling. Battery manufacturers typically aim to sustain a mix temperature of 55–60°C by adjusting the acid flow rate with cooling assistance. Initially, the paste consisted of 3BS, which was later converted to 4BS during higher-temperature curing profiles.
Factors affecting pasting process
Following paste mixing, the paste was transferred to the pasting hopper and then applied to the grids. A flash drying oven was employed to ensure a pasted plate temperature above 50°C.This initiates the curing reaction prior to loading into the chamber, and prevents plates sticking together on the skid.
3BS sulfate pastes exhibited better pastability than 4BS ones. The 4BS pastes resulted from aggressive addition of acid, resulting in higher temperature zones during the paste mix.
Delays in pasting may lead to moisture loss, which affects the next process of curing, resulting in plates with a higher post-curing residual lead content. For positive plates, cured free lead levels need to be maintained below 2%, and for negative plates, below 5%.
The pasted plates, released from the hopper, were then conveyed into the oven. Consequently, the oven temperature and chain speed were critical factors. Before the curing process, the recommended moisture content after flash drying should be maintained between 11 and 8.5% for effective curing.
Experiment description
In the experiment, positive and negative plates were prepared using a common procedure. The positive plates underwent two different curing profiles: LTCP and HTCP. The curing profile for the negative plates remained unchanged. After production, the plates were extensively analysed using chemical and XRD methods. Once assembled, batteries were created using both LTCP plates and HTCP plates, with common negative plates. The batteries were formed using an acid circulation system, followed by a gel forming circulation process and gel conditioning charging.
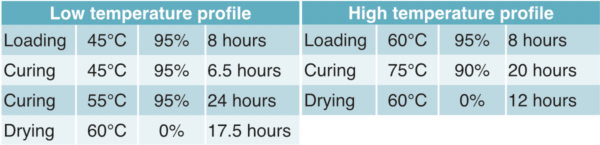
Positive plate preparation
Positive plates were manufactured using 1,000Kgs of ball mill grey oxide to which is added 12% of demineralised water, 8.33% of pure sulfuric acid, and 2% of fibre expressed as percentages of 1,000Kgs of grey oxide.
Negative plate preparation:
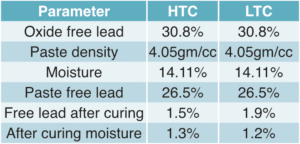
Negative plates were produced using 1,000Kgs of ball mill grey oxide with 13.5% additives, including barium sulfate, Vanisperse-A, and carbon black, along with 10% water and 4.65% pure sulfuric acid expressed as percentages of 1,000kgs of grey oxide.
For both sets of plates the peak mixing temperature was set at 55°C, and acid addition was controlled to maintain the mixture temperature. Pb-Ca grids were used for pasting.
XRD and SEM tests
A spatula was used to remove the pasted active materials from the cured plates, and place them in the sample holder. An XRD test was initiated using a Malvern Panalytical diffractometer.
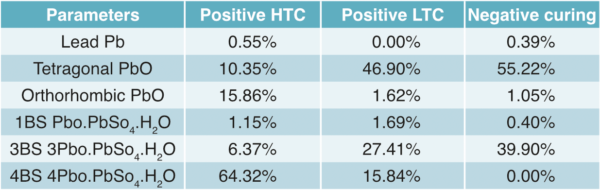
From the results shown in Table 4, it was evident that the HTCP plates had a higher 4BS content compared to the LTCP plates.
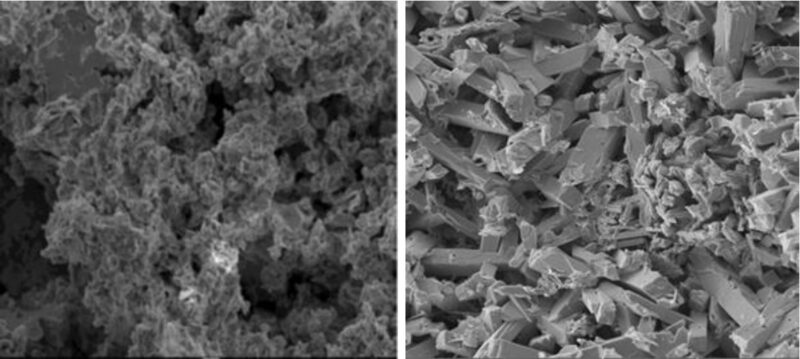
SEM results were obtained from Black Diamond Structure’s R&D Laboratory. In the SEM images, it was evident that HTCP plates had a more pronounced crystal structure due to having more of the larger 4BS crystals than LTCP plates (Fig 3).
Cured plate comparisons
In the manufacturing process, we did not send all of the samples for SEM analysis to third parties due to time constraints. Therefore, internally, we introduced a corrosion layer thickness procedure to evaluate the curing performance of the negative and positive plates. We also measured the porosity, the amount of residual lead, and the moisture content.
The corrosion layer thickness was 7-8 microns for the HTC plate compared to 4-5 microns for both the LTC and negative plates. Also for the HTC cured plate, the porosity was 6% higher, whilst the residual lead and humidity were significantly lower than the values measured in the LTC and negative plates.
Assembly of batteries
Batteries were made with low-temperature-cured positive plates and high-temperature-cured plates. They were assembled with four positive and five negative plates in each cell. The negative plate was common for both trials.
Charging of batteries
All batteries were formed using an acid-circulation-system formation module. The initial gravity of the acid was 1.110g/cc at 25°C temperature. During the two-hour soaking the acid temperature was maintained using the cooling tower to avoid high temperatures and keep the cell temperature between 40–50°C (Fig 4). Filling of the acid took 15–20 minutes, dependent on the volume of the batteries, and for the rest of the time, the acid was continuously circulated through a cooling tower to keep the acid temperature down.
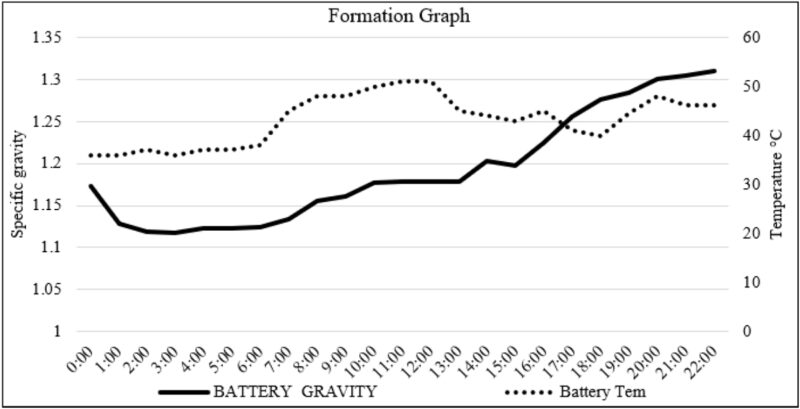
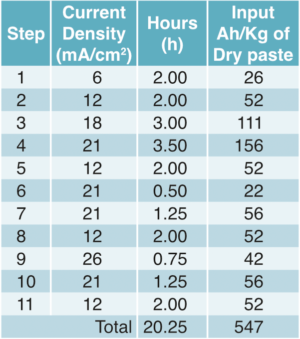
During the formation schedule (Table 5), from the starting value of the original 1.18g/cm3, the acid density gradually increased from steps one to 11. During the 11th step of charging, cells were adjusted to a final density of acid 1.30g/cm3.
After formation, we checked the acid density of each cell. Once the gravity reached 1.300g/cm3, those batteries were discharged at the C5 rate in the formation apparatus without acid circulation. The end of discharge criteria were fixed at 10.2V and a final acid density of 1.130 ±0.005 gm/cm3. Once all batteries reached 1.130g/cm3 density, then the circuit was disconnected by the auto mode commands.
After this, all of the batteries’ acid was exchanged with 6% fumed silica and 1.130g/cm3 acid in a gel mixing and acid exchange machine. This exchange process took 2 hours. After gel electrolyte circulation, silica samples were taken from the cells randomly, and the chemical composition was checked and confirmed. During this gel electrolyte circulation, the mixed silica acid temperature was maintained below 10°C. The batteries then stood for 24 hours at the ambient room temperature.
Once room temperature was reached, all batteries were connected to the rectifier for the gel conditioning charging. The charging input was 1.25 times the discharged Ah. At the end of charging, the gel had solidified. The trial batteries were sent for capacity and 70% DoD cycling.
Electrical Test
We tested both batteries following the guidelines outlined in IEC 60254-1 for 70% DoD. According to the standard, we initiated the battery discharge at 10.8A for 3.5 hours. After every 49 cycles, we conducted a capacity test. If the capacity fell below 80%, we terminated the battery testing.
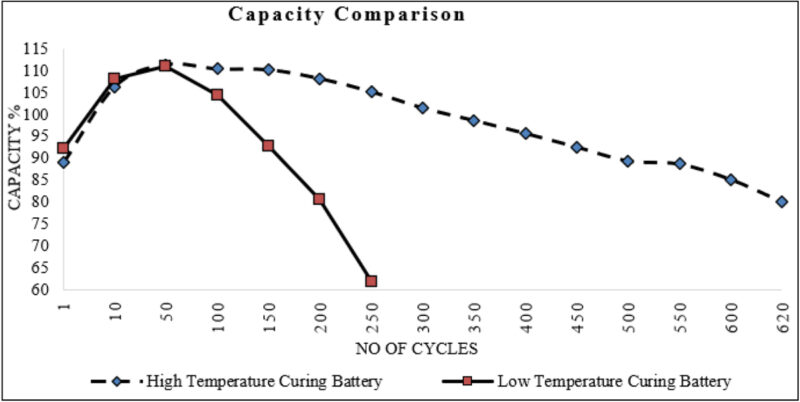
The high-temperature-cured battery performed well in the electrical test results, achieving more than 620 cycles. However, the low-temperature-cured battery exhibited poor performance, reaching only 200 cycles before falling below 80% capacity (Fig 5).
Formed plate PbO2 analysis
After neutralising the formed plate in demineralised water, it was dried at 60°C and ground. After that, the sample was passed through a 150µm sieve. The PbO2 content was determined by a laboratory titration method.
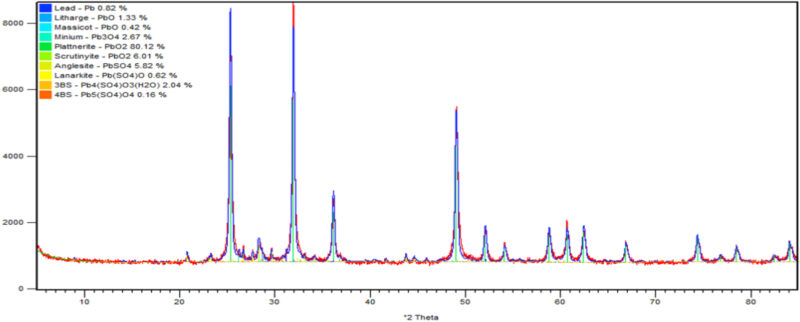
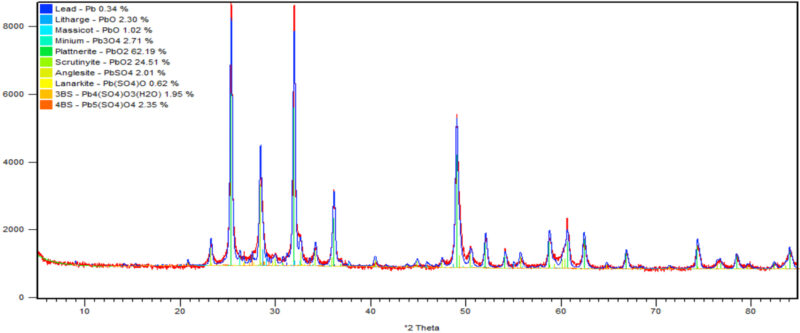
Further positive plates were washed with demineralised water until the pH of the plate reached 6–7. The plate was dried at 60°C for 12 hours and was sent for XRD and SEM analysis. The results are shown in Figs 6 & 7.
Result and Discussion
XRD analysis showed that the negative cured plates had a mostly 3BS structure (39%) with no 4BS. The low temperature cured positive plates had the lowest 4BS at 15.8% and a corresponding 3BS of 27%. This compares with the high temperature curing process having the highest 4BS and lowest 3BS at 64.3% and 6.4% respectively. Regarding the formed plates, both positives contained around 86% PbO2. The high temperature cured plate contained the highest proportion of plattnerite (β-PbO2) at 80%, (Fig 6). This can be compared to 62% for the low temperature cure, (Fig 7).
The SEM analysis of the cured positive plates revealed a larger crystalline structure of 30 to 40 microns for the higher temperature compared to that of 2–3 microns for the lower temperature cured plates (Fig 3) In both cases the individual crystals of the structures were reasonably uniform in size.
This study highlighted the significant impact of the curing temperature algorithm on the lifecycle performance of gelled valved-regulated lead-acid batteries. The high-temperature curing profile (HTCP) resulted in a deeper grid corrosion layer. As a result, there was improved active material adhesion and cohesion. This contributed to the enhanced cycling performance compared to the low-temperature curing profile (LTCP). The HTCP plates achieved 620 70% DoD cycles to failure, compared to 200 for the LTCP plates.
If lead-acid battery manufacturers were to optimise the curing process by adopting a higher temperature curing algorithm, it could improve their battery deep-cycle life. This research has provided valuable insights for the lead-acid battery industry. These will contribute to the development of the most effective and durable, gelled, valve-regulated, lead-acid batteries.