The Battery Recycling Group at the University of Warwick’s WMG department has successfully developed an improved method for lithium recovery from end-of-life lithium-ion batteries, it said.
It uses a carbothermal reduction process and the researchers said it is able to recover more than 90% of the lithium at a purity of over 99%.
In a paper published in Science Direct, the UK researchers said their work presents an improved carbothermal reduction combined with a water leaching process for lithium recovery from Li(NixMnyCo1-x-y)O2 cathode materials.
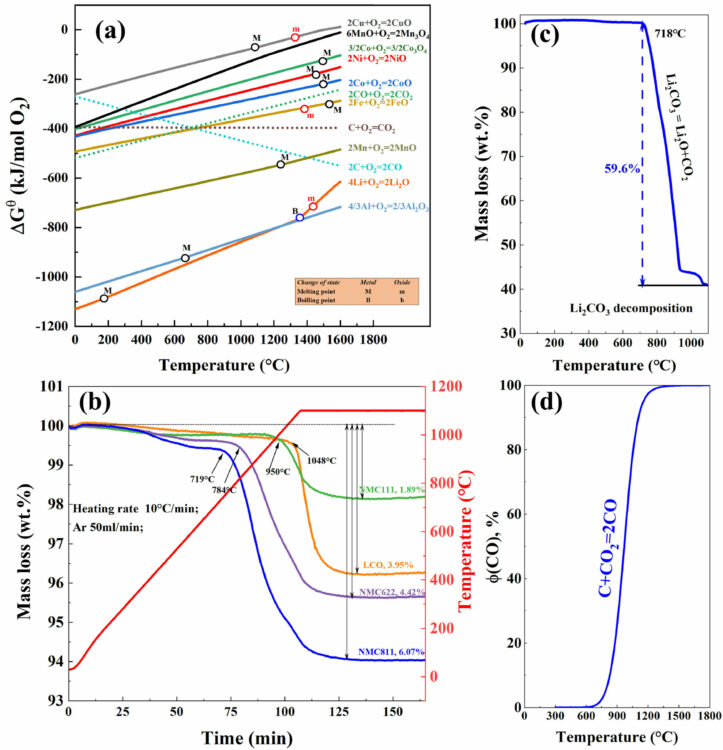
Results show that Co and Ni are reduced to metal, Mn remains as an oxide, whilst Li is converted mainly into Li2CO3 at temperatures lower than 800℃ and Li2O when the temperature exceeds 900℃.
Water leaching was used to efficiently extract lithium using low liquid-solid ratios. The team said this improved lithium extraction process can effectively recover more than 93% of lithium as lithium hydroxide or carbonate at a purity greater than 99.5%.
The effect of aluminium and copper impurities on the lithium recovery rate was investigated. They found that copper has no significant effect on the lithium recovery rate. The presence of aluminium decreases the lithium recovery rate through the production of lithium aluminate, however.
Fig. 1. (a) Ellingham diagram for several metals in Li-batteries, (b) TGA-DSC result for pyrolysis of Li(NixMnyCo1-x-y)O2 in argon, (c) TGA result for decomposition of Li2CO3 in argon, and (d) equilibrium diagram of CO partial pressure for the Boudouard reaction.